Osmosis is the movement of water molecules through a semipermeable membrane from an area of low solute concentration to high solute concentration, balancing fluid levels inside and outside cells. This vital biological process supports hydration, nutrient absorption, and waste removal in living organisms. Explore the rest of the article to understand how osmosis impacts your body's essential functions and overall health.
Table of Comparison
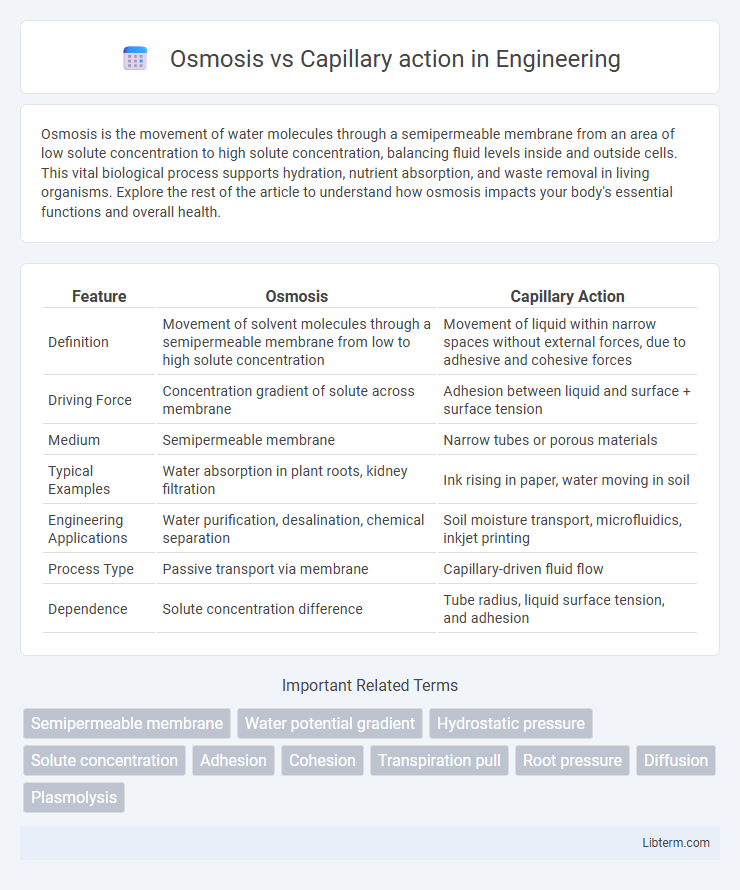
| Feature | Osmosis | Capillary Action |
|---|---|---|
| Definition | Movement of solvent molecules through a semipermeable membrane from low to high solute concentration | Movement of liquid within narrow spaces without external forces, due to adhesive and cohesive forces |
| Driving Force | Concentration gradient of solute across membrane | Adhesion between liquid and surface + surface tension |
| Medium | Semipermeable membrane | Narrow tubes or porous materials |
| Typical Examples | Water absorption in plant roots, kidney filtration | Ink rising in paper, water moving in soil |
| Engineering Applications | Water purification, desalination, chemical separation | Soil moisture transport, microfluidics, inkjet printing |
| Process Type | Passive transport via membrane | Capillary-driven fluid flow |
| Dependence | Solute concentration difference | Tube radius, liquid surface tension, and adhesion |
Introduction to Osmosis and Capillary Action
Osmosis is the movement of water molecules across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration, crucial in biological and chemical processes. Capillary action refers to the ability of a liquid to flow in narrow spaces without external forces, driven by adhesive and cohesive forces between the liquid and surrounding surfaces. Both phenomena are fundamental in water transport in plants and various industrial applications.
Defining Osmosis: Core Principles
Osmosis is the passive movement of water molecules through a semipermeable membrane from a region of low solute concentration to a region of high solute concentration, driven by osmotic pressure. It plays a critical role in biological processes such as nutrient absorption and cellular hydration. Unlike capillary action, which involves water movement through narrow spaces against gravity due to adhesion and cohesion, osmosis specifically depends on solute concentration gradients and membrane permeability.
Capillary Action Explained
Capillary action occurs when liquid spontaneously rises or moves within narrow spaces due to the adhesive force between the liquid and solid surfaces and the cohesive forces within the liquid itself. This phenomenon is critical in natural processes such as water transport in plants, where xylem vessels act as tiny capillaries drawing water upward against gravity. Unlike osmosis, which involves the selective movement of water across semipermeable membranes driven by concentration gradients, capillary action relies on surface tension and intermolecular interactions without the need for membrane permeability.
Key Differences Between Osmosis and Capillary Action
Osmosis is the movement of water molecules through a semi-permeable membrane from a region of low solute concentration to high solute concentration, driven by concentration gradients. Capillary action involves the movement of liquid within narrow spaces without external forces, primarily influenced by cohesive and adhesive forces between the liquid and solid surfaces. The key difference lies in osmosis requiring a semi-permeable membrane and solute concentration gradient, whereas capillary action depends on surface tension and does not involve membranes.
Molecular Mechanisms Behind Osmosis
Osmosis involves the movement of water molecules across a semipermeable membrane from a region of low solute concentration to high solute concentration, driven by the chemical potential gradient and the membrane's selective permeability. Water molecules diffuse through the membrane by transiently forming hydrogen bonds while the membrane restricts solute passage, maintaining the osmotic pressure difference. This molecular mechanism contrasts with capillary action, where adhesion and cohesion forces between water molecules and solid surfaces move liquid without selective molecular permeability.
The Physics of Capillary Action
Capillary action occurs due to the adhesion between liquid molecules and the walls of narrow tubes combined with cohesive forces within the liquid, causing it to rise against gravity. The height to which a liquid climbs in a capillary tube is governed by the balance of surface tension, liquid density, gravitational acceleration, and tube radius, described mathematically by the Jurin's law. This phenomenon plays a critical role in plant water transport and microfluidic device operation, where precise control of fluid movement is essential.
Real-World Examples of Osmosis
Osmosis is the process where water molecules move across a semipermeable membrane from a region of low solute concentration to high solute concentration, commonly seen in plant roots absorbing water from soil. This mechanism is crucial in medical dialysis treatments, where osmosis helps remove waste products from the blood through a synthetic membrane. Unlike capillary action, which involves liquid rising in narrow spaces due to adhesive and cohesive forces, osmosis specifically depends on solute concentration gradients for water movement.
Practical Applications of Capillary Action
Capillary action enables water transport in plants, allowing nutrients to reach leaves and sustain growth, which is crucial for agriculture and horticulture. It is also essential in medical devices like thin-layer chromatography and blood sample collection, ensuring precise fluid movement without external forces. Furthermore, capillary action supports ink circulation in pens and enhances moisture wicking in fabrics, demonstrating its broad impact across everyday technologies.
Osmosis vs Capillary Action: Comparative Table
Osmosis involves the movement of water molecules through a semipermeable membrane from a lower solute concentration to a higher solute concentration, crucial for cellular processes and nutrient absorption. Capillary action occurs when liquid rises in narrow tubes due to adhesive and cohesive forces, essential in plant water transport and ink flow in pens. A comparative table highlights osmosis based on selective permeability and solute gradients, while capillary action is driven by surface tension and tube diameter without membrane involvement.
Summary: Choosing the Right Concept for Your Study
Osmosis involves the movement of water molecules through a semipermeable membrane from low to high solute concentration, crucial for cellular processes and fluid balance studies. Capillary action describes the ability of liquid to flow in narrow spaces without external forces, important in soil water movement and plant physiology research. Selecting between osmosis and capillary action depends on the study's focus--membrane permeability and solute gradients favor osmosis, whereas liquid adhesion and cohesion in confined spaces highlight capillary action.
Osmosis Infographic
 libterm.com
libterm.com