Surface tension is the cohesive force at the surface of a liquid that causes it to behave like a stretched elastic membrane, allowing it to resist external force. This phenomenon enables small objects to float on water without sinking and plays a crucial role in processes like capillary action and droplet formation. Explore the rest of the article to understand how surface tension impacts your daily life and various scientific applications.
Table of Comparison
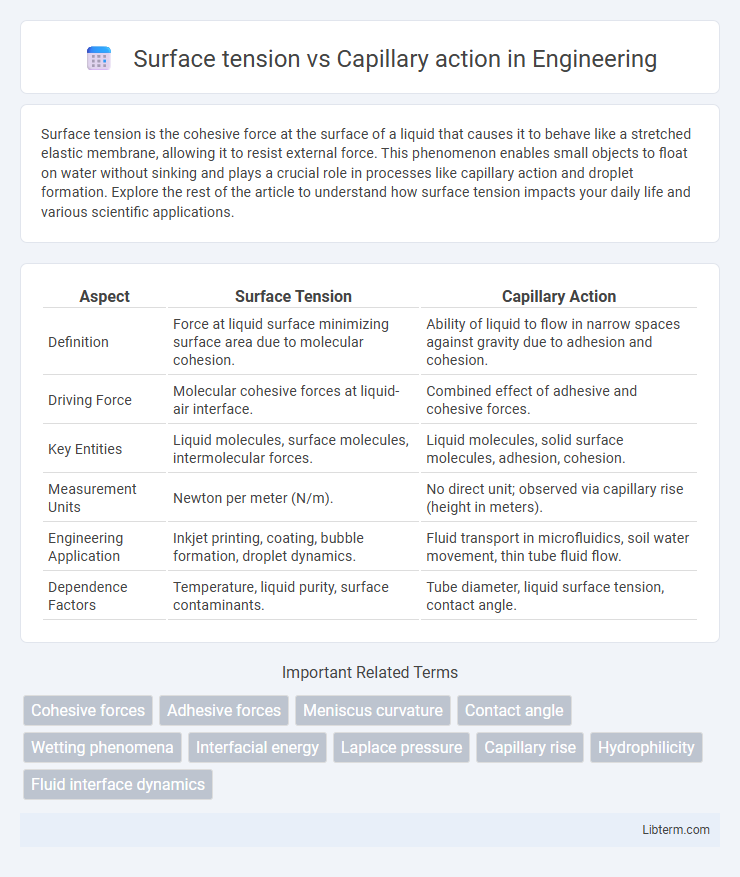
| Aspect | Surface Tension | Capillary Action |
|---|---|---|
| Definition | Force at liquid surface minimizing surface area due to molecular cohesion. | Ability of liquid to flow in narrow spaces against gravity due to adhesion and cohesion. |
| Driving Force | Molecular cohesive forces at liquid-air interface. | Combined effect of adhesive and cohesive forces. |
| Key Entities | Liquid molecules, surface molecules, intermolecular forces. | Liquid molecules, solid surface molecules, adhesion, cohesion. |
| Measurement Units | Newton per meter (N/m). | No direct unit; observed via capillary rise (height in meters). |
| Engineering Application | Inkjet printing, coating, bubble formation, droplet dynamics. | Fluid transport in microfluidics, soil water movement, thin tube fluid flow. |
| Dependence Factors | Temperature, liquid purity, surface contaminants. | Tube diameter, liquid surface tension, contact angle. |
Introduction to Surface Tension and Capillary Action
Surface tension is a physical phenomenon arising from the cohesive forces between liquid molecules at the surface, creating a "skin-like" effect that allows objects to float or insects to walk on water. Capillary action occurs when adhesive forces between a liquid and a solid surface, combined with surface tension, cause the liquid to rise or fall within narrow tubes or porous materials. Understanding the interplay between surface tension and capillary action is essential in fields such as biology, chemistry, and engineering for explaining fluid behavior in natural and artificial systems.
Defining Surface Tension: Key Concepts
Surface tension is the cohesive force at the surface of a liquid that causes it to behave like a stretched elastic membrane. It arises from the imbalance of intermolecular forces experienced by molecules at the liquid-air interface, leading to minimized surface area. This phenomenon is crucial for understanding capillary action, where surface tension enables liquids to flow in narrow spaces against gravity.
Understanding Capillary Action: Basics Explained
Capillary action occurs when liquid spontaneously rises or falls within a narrow tube due to the interplay between cohesive forces of the liquid and adhesive forces between the liquid and tube surface. Surface tension, a key factor in this process, is the elastic tendency of liquids caused by molecular attraction, which helps the liquid resist external force. Understanding this balance explains why water can climb thin glass tubes, defying gravity through the combined effects of adhesion, cohesion, and surface tension.
Molecular Forces Behind Surface Tension
Surface tension arises from cohesive molecular forces, primarily hydrogen bonding, pulling liquid molecules at the surface inward to minimize surface area, creating a "skin-like" effect. Capillary action results from the balance between these cohesive forces and adhesive forces between the liquid and surrounding solid surfaces, enabling liquid to travel upward against gravity in narrow tubes. The strength of surface tension is directly linked to the intensity of intermolecular forces, influencing how liquids interact with their environments at the molecular level.
How Capillary Action Works at the Micro Level
Capillary action occurs due to the adhesive forces between a liquid and the surrounding solid surfaces overcoming the cohesive forces within the liquid, causing the liquid to rise or fall in narrow spaces. At the micro level, intermolecular attractions pull the liquid molecules upward along the surface of tiny tubes or porous materials, counteracting gravity. This phenomenon is critical in processes like water transport in plants and ink flow in pens, where surface tension and adhesion interact to drive fluid movement.
Surface Tension in Everyday Life
Surface tension, caused by cohesive forces between liquid molecules, allows insects like water striders to walk on water surfaces and enables droplets to maintain a nearly spherical shape, reducing surface area. It plays a crucial role in the formation of bubbles and the behavior of liquids in small containers or porous materials. This phenomenon impacts everyday activities such as cleaning, where detergents reduce surface tension to improve the wetting of fabrics and surfaces.
Real-World Examples of Capillary Action
Capillary action enables water to move through narrow spaces against gravity, as observed when water rises in plant xylem vessels to nourish leaves or when ink flows smoothly through the tiny channels of a fountain pen. This phenomenon also plays a critical role in soil moisture retention, allowing roots to access water trapped in microscopic soil pores. In contrast, surface tension involves the cohesive forces at a liquid's surface, responsible for water droplets maintaining shape and small insects walking on water without sinking.
Surface Tension vs Capillary Action: Main Differences
Surface tension is the cohesive force between liquid molecules at the surface, causing the liquid to behave as an elastic sheet, while capillary action is the ability of a liquid to flow in narrow spaces without external forces due to adhesive and cohesive forces. Surface tension primarily influences phenomena like water droplets forming spheres, whereas capillary action enables liquids to move through thin tubes or porous materials, such as in plant xylem or paper towels. The key difference lies in surface tension being a surface phenomenon caused by molecular attraction, whereas capillary action results from the interplay of adhesion between liquid and solid surfaces with cohesion within the liquid.
Factors Affecting Surface Tension and Capillary Action
Surface tension is influenced by temperature, with increased temperatures reducing molecular cohesion and thus lowering surface tension, while the presence of surfactants disrupts intermolecular forces and decreases surface tension significantly. Capillary action depends on the adhesive forces between the liquid and the surface, the diameter of the capillary tube, and the liquid's surface tension; smaller diameters and higher surface tension enhance capillary rise. The contact angle between the liquid and solid surface also plays a crucial role, where a low contact angle promotes stronger capillary action due to greater adhesive attraction.
Practical Applications and Impacts in Science and Industry
Surface tension enables the formation of droplets and bubbles critical for inkjet printing and pesticide spraying, while capillary action drives fluid movement in porous materials, essential for oil recovery and soil irrigation. In medical diagnostics, surface tension influences blood droplet formation on test strips, whereas capillary action allows for sample wicking in microfluidic devices. Both phenomena impact material science by enhancing coatings' adhesion and enabling precise liquid transport in lab-on-a-chip technologies.
Surface tension Infographic
 libterm.com
libterm.com