Corrosion is the gradual degradation of materials, usually metals, due to chemical reactions with their environment, leading to weakened structures and potential failure. Understanding the causes, types, and prevention methods of corrosion is essential for protecting your assets and ensuring safety. Explore the rest of this article to learn how to effectively combat corrosion and extend the life of your materials.
Table of Comparison
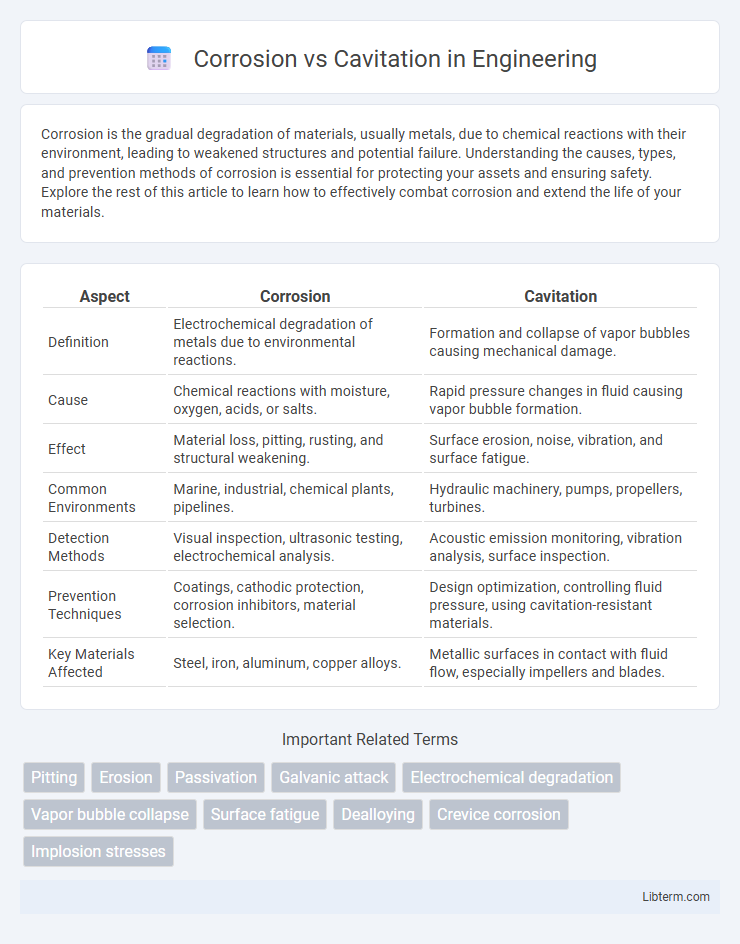
| Aspect | Corrosion | Cavitation |
|---|---|---|
| Definition | Electrochemical degradation of metals due to environmental reactions. | Formation and collapse of vapor bubbles causing mechanical damage. |
| Cause | Chemical reactions with moisture, oxygen, acids, or salts. | Rapid pressure changes in fluid causing vapor bubble formation. |
| Effect | Material loss, pitting, rusting, and structural weakening. | Surface erosion, noise, vibration, and surface fatigue. |
| Common Environments | Marine, industrial, chemical plants, pipelines. | Hydraulic machinery, pumps, propellers, turbines. |
| Detection Methods | Visual inspection, ultrasonic testing, electrochemical analysis. | Acoustic emission monitoring, vibration analysis, surface inspection. |
| Prevention Techniques | Coatings, cathodic protection, corrosion inhibitors, material selection. | Design optimization, controlling fluid pressure, using cavitation-resistant materials. |
| Key Materials Affected | Steel, iron, aluminum, copper alloys. | Metallic surfaces in contact with fluid flow, especially impellers and blades. |
Understanding Corrosion: Definition and Types
Corrosion is the electrochemical deterioration of metals caused by chemical reactions with their environment, leading to material loss and structural damage. Common types include uniform corrosion, galvanic corrosion, pitting corrosion, crevice corrosion, and stress corrosion cracking, each varying by mechanism and impact severity. Understanding these types helps in selecting appropriate materials and protective measures for industrial applications.
What is Cavitation? Key Concepts Explained
Cavitation is the formation and collapse of vapor bubbles in a liquid due to rapid changes in pressure, often occurring near pump impellers, propeller blades, or other hydraulic equipment. This phenomenon causes localized shock waves and high temperatures, leading to material erosion and surface pitting distinct from corrosion, which is a chemical or electrochemical reaction causing gradual material degradation. Understanding cavitation involves recognizing the role of fluid dynamics and pressure fluctuations that generate these destructive vapor bubbles.
Causes of Corrosion in Industrial Systems
Corrosion in industrial systems primarily results from chemical reactions between metals and environmental elements such as oxygen, moisture, acids, and salts, leading to material degradation. Factors like exposure to harsh chemicals, high temperatures, and varying pH levels accelerate corrosion processes in pipelines, tanks, and machinery. In contrast, cavitation involves physical damage caused by vapor bubble collapse, which does not directly cause corrosion but can exacerbate existing corrosion through surface erosion.
Triggers and Mechanisms of Cavitation
Cavitation occurs when vapor bubbles form and collapse in a liquid due to local pressure dropping below the vapor pressure, commonly triggered by rapid fluid velocity changes or flow obstructions in pumps and propellers. This phenomenon involves mechanical damage caused by the implosion of vapor pockets, which generates high-pressure shock waves and microjets impacting metal surfaces. Unlike corrosion, which is an electrochemical process resulting from chemical reactions between metals and their environment, cavitation damage is primarily physical and results from dynamic fluid forces.
Visual and Structural Differences: Corrosion vs Cavitation
Corrosion typically appears as uniform rusting or pitting on metal surfaces, often accompanied by discoloration and flaky deposits, which indicate chemical degradation. Cavitation causes distinctive structural damage characterized by small, crater-like pits and surface erosion resulting from rapid vapor bubble collapse, leading to a rough, uneven texture. While corrosion affects the metal's chemical integrity, cavitation primarily causes mechanical surface fatigue and localized material loss.
Impact of Corrosion on Equipment Longevity
Corrosion accelerates metal degradation by chemical or electrochemical reactions, significantly reducing equipment longevity through pitting, cracking, and material loss. This deterioration compromises structural integrity, leading to unexpected failures and increased maintenance costs in industrial systems. Effective corrosion prevention methods like coatings, cathodic protection, and material selection are essential to extend the lifespan of machinery and infrastructure.
Cavitation’s Effects on System Performance
Cavitation causes the formation and implosion of vapor bubbles in a fluid, leading to localized high-pressure shock waves that erode metal surfaces and degrade components such as pumps, propellers, and turbines. This erosion reduces system efficiency by increasing surface roughness and causing flow disruptions, resulting in higher energy consumption and premature equipment failure. Continuous cavitation damage can lead to costly maintenance, unscheduled downtime, and a significant decrease in overall system reliability.
Detection and Diagnosis: Identifying Corrosion or Cavitation
Detection and diagnosis of corrosion or cavitation rely on distinct techniques suited to their unique signatures. Corrosion is typically identified using visual inspection, ultrasonic thickness measurements, and corrosion sensors that detect material degradation and chemical changes. Cavitation diagnosis involves acoustic emission monitoring, vibration analysis, and high-speed imaging to capture bubble formation and collapse, distinguishing it from corrosion damage patterns.
Prevention Strategies for Corrosion and Cavitation
Effective prevention strategies for corrosion include applying protective coatings, using corrosion inhibitors, and selecting corrosion-resistant materials such as stainless steel or titanium. Cavitation prevention focuses on optimizing fluid dynamics by reducing flow velocity, improving pump and valve design, and maintaining proper system pressure to avoid vapor bubble formation. Combining corrosion-resistant materials with cavitation control techniques significantly extends the lifespan of equipment in marine, industrial, and hydraulic applications.
Choosing the Right Protection: Corrosion-Resistant vs Anti-Cavitation Solutions
Choosing the right protection involves understanding that corrosion-resistant coatings primarily prevent chemical degradation caused by environmental factors like moisture and chemicals, while anti-cavitation solutions focus on mitigating physical damage from bubble collapse in fluids. Materials such as stainless steel and epoxy coatings excel in corrosion resistance, but anti-cavitation protection often requires specialized elastomers or hard coatings like tungsten carbide to withstand high-impact erosion. Selecting between these solutions depends on the operational environment, with corrosion resistance favored in chemically aggressive contexts and anti-cavitation treatments essential for hydraulic components exposed to vapor bubble implosions.
Corrosion Infographic
 libterm.com
libterm.com