Melting point depression occurs when the melting point of a substance is lowered due to the presence of impurities or solutes, disrupting the solid lattice structure. This phenomenon is essential in applications such as antifreeze formulation and food preservation, where control over phase changes is critical. Discover how understanding melting point depression can enhance Your grasp of material properties by reading the rest of the article.
Table of Comparison
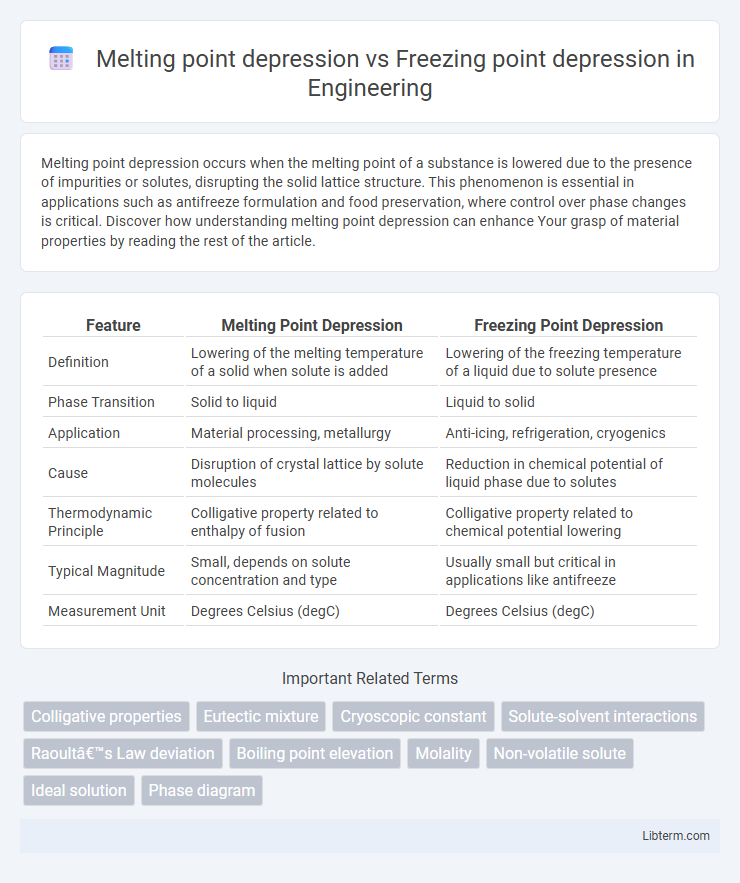
| Feature | Melting Point Depression | Freezing Point Depression |
|---|---|---|
| Definition | Lowering of the melting temperature of a solid when solute is added | Lowering of the freezing temperature of a liquid due to solute presence |
| Phase Transition | Solid to liquid | Liquid to solid |
| Application | Material processing, metallurgy | Anti-icing, refrigeration, cryogenics |
| Cause | Disruption of crystal lattice by solute molecules | Reduction in chemical potential of liquid phase due to solutes |
| Thermodynamic Principle | Colligative property related to enthalpy of fusion | Colligative property related to chemical potential lowering |
| Typical Magnitude | Small, depends on solute concentration and type | Usually small but critical in applications like antifreeze |
| Measurement Unit | Degrees Celsius (degC) | Degrees Celsius (degC) |
Introduction to Melting Point and Freezing Point Depression
Melting point depression occurs when the addition of a solute lowers the temperature at which a solid becomes a liquid, whereas freezing point depression refers to the decrease in the temperature at which a liquid turns into a solid due to the presence of dissolved substances. Both phenomena are colligative properties, dependent on solute particle concentration rather than chemical identity, influencing phase transitions in solutions. Understanding these effects is crucial for applications in cryoprotection, antifreeze formulations, and material science.
Defining Melting Point Depression
Melting point depression refers to the lowering of a substance's melting temperature when a solute is dissolved in a solvent, disrupting the solid phase's structural order. This phenomenon contrasts with freezing point depression, which specifically describes the lowering of the temperature at which a liquid becomes solid. Melting point depression is crucial in characterizing the purity of compounds and understanding phase transitions in solutions.
Defining Freezing Point Depression
Freezing point depression refers to the lowering of the temperature at which a liquid solidifies due to the presence of a solute, disrupting the formation of a solid lattice. Melting point depression describes the decrease in melting temperature of a solid when impurities or solutes are added, affecting its molecular structure. Both phenomena result from colligative properties, but freezing point depression specifically applies to the solidification of liquids.
Key Differences Between Melting Point and Freezing Point Depression
Melting point depression refers to the lowering of a solid's melting temperature when a solute is added, while freezing point depression specifically describes the reduction in the temperature at which a liquid solidifies. Both phenomena occur due to solute particles disrupting the solvent's crystal lattice formation, but melting point depression applies to the transition from solid to liquid, and freezing point depression applies to the transition from liquid to solid. Key differences include the phase transition direction and the practical applications, such as antifreeze using freezing point depression to prevent ice formation.
Thermodynamic Principles Involved
Melting point depression and freezing point depression both arise from colligative properties governed by thermodynamic principles involving the disruption of phase equilibrium. When a non-volatile solute is dissolved in a solvent, the chemical potential of the liquid phase decreases, lowering the melting point by shifting the solid-liquid equilibrium. This effect is quantitatively described by the Clausius-Clapeyron equation and Raoult's law, reflecting changes in Gibbs free energy and vapor pressure associated with solute-solvent interactions.
Role of Solutes in Depression Effects
Solutes disrupt the orderly lattice formation of solvents, leading to melting point depression by reducing the temperature at which a solid becomes liquid. In freezing point depression, solutes lower the temperature at which a liquid solidifies by interfering with crystal nucleation and growth. Both effects are quantitatively described by colligative properties, where solute concentration directly influences the degree of temperature depression.
Practical Applications in Everyday Life
Melting point depression and freezing point depression both involve the lowering of phase transition temperatures due to the presence of solutes, with freezing point depression being crucial in de-icing roads using salt to prevent ice formation. In everyday life, freezing point depression is applied in antifreeze solutions for car engines, enabling fluids to remain liquid below 0degC and thus preventing engine damage in cold climates. Melting point depression plays a role in chocolate manufacturing, where controlled melting behaviors enhance texture and mouthfeel by regulating fat and sugar content.
Experimental Methods for Measurement
Melting point depression and freezing point depression are experimentally measured using methods such as differential scanning calorimetry (DSC) and cryoscopic techniques. DSC provides precise determination by monitoring heat flow associated with phase transitions, enabling accurate detection of temperature shifts caused by solute presence. Cryoscopy involves measuring the lowering of a solvent's freezing point through controlled cooling, often using a calibrated thermistor or thermocouple, to quantify colligative properties.
Common Examples in Chemistry and Industry
Melting point depression and freezing point depression both describe the lowering of phase transition temperatures due to solutes, but melting point depression is typically observed in solid mixtures such as alloys or pharmaceutical compounds, while freezing point depression is common in solutions like antifreeze in automotive cooling systems and salt on icy roads. In chemistry, melting point depression is exploited in the purification of compounds and determining purity, whereas freezing point depression is utilized in industries to prevent ice formation and stabilize food products during freezing. Notable examples include the use of sodium chloride to lower the freezing point of water for de-icing and the blending of metal alloys like solder, which melts at lower temperatures than its constituents.
Summary and Future Research Directions
Melting point depression and freezing point depression both describe the lowering of phase transition temperatures due to solute presence, with melting point depression referring to the solid-to-liquid transition and freezing point depression to the liquid-to-solid transition. Current research emphasizes the molecular mechanisms driving these phenomena in complex mixtures, such as ionic liquids and nanofluids, revealing distinct thermodynamic behaviors influenced by solute-solvent interactions. Future studies will likely explore advanced computational models and nanoscale measurements to better predict phase behavior in multi-component systems, enhancing applications in cryopreservation, materials science, and chemical engineering.
Melting point depression Infographic
 libterm.com
libterm.com