A polytropic line represents a thermodynamic process where the pressure and volume of a gas follow the relation \( PV^n = \text{constant} \), with \( n \) being the polytropic index. This concept is widely used to model processes such as compression and expansion in engines, where heat transfer occurs but not in a strictly adiabatic or isothermal manner. Explore the rest of this article to understand how the polytropic line applies to your engineering calculations and system analyses.
Table of Comparison
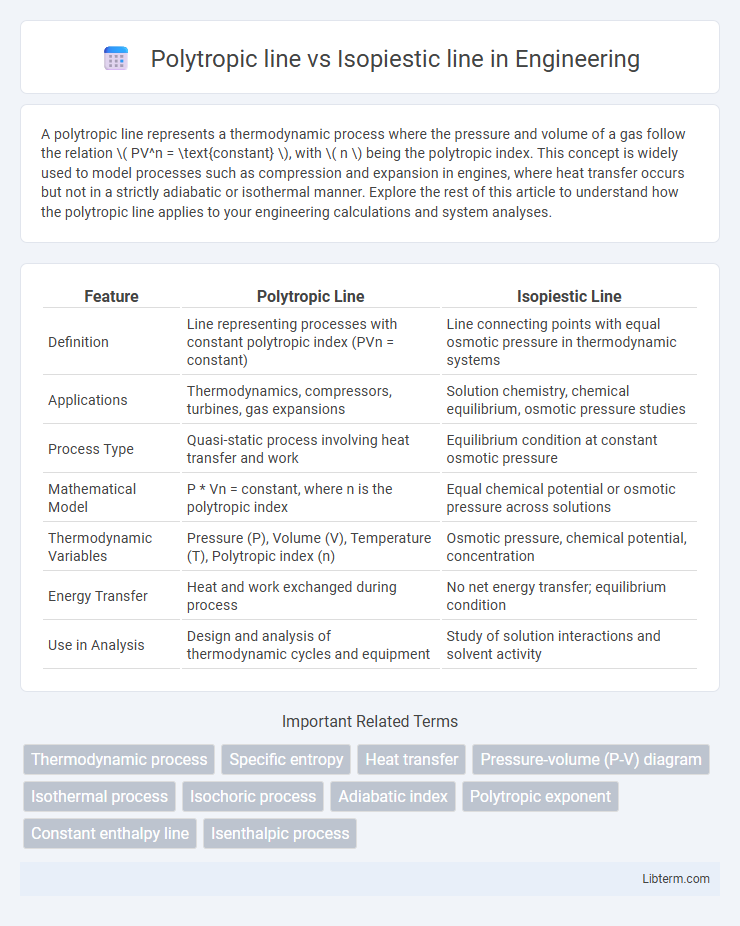
| Feature | Polytropic Line | Isopiestic Line |
|---|---|---|
| Definition | Line representing processes with constant polytropic index (PVn = constant) | Line connecting points with equal osmotic pressure in thermodynamic systems |
| Applications | Thermodynamics, compressors, turbines, gas expansions | Solution chemistry, chemical equilibrium, osmotic pressure studies |
| Process Type | Quasi-static process involving heat transfer and work | Equilibrium condition at constant osmotic pressure |
| Mathematical Model | P * Vn = constant, where n is the polytropic index | Equal chemical potential or osmotic pressure across solutions |
| Thermodynamic Variables | Pressure (P), Volume (V), Temperature (T), Polytropic index (n) | Osmotic pressure, chemical potential, concentration |
| Energy Transfer | Heat and work exchanged during process | No net energy transfer; equilibrium condition |
| Use in Analysis | Design and analysis of thermodynamic cycles and equipment | Study of solution interactions and solvent activity |
Introduction to Thermodynamic Processes
Polytropic lines represent processes where the heat capacity ratio varies with pressure and volume, following the equation \(PV^n = \text{constant}\), essential for modeling real gas behavior in thermodynamics. Isopiestic lines, on the other hand, depict states of equal vapor pressure, crucial for phase equilibrium analysis and osmotic studies. Understanding the distinctions between polytropic and isopiestic lines aids in accurately describing energy transfer and phase changes in thermodynamic systems.
Defining the Polytropic Line
The polytropic line represents a process in thermodynamics where the pressure and volume of a gas follow the relation \(P V^n = \text{constant}\), with \(n\) being the polytropic index that characterizes heat transfer during the process. This line differs from the isopiestic line, which pertains to constant osmotic pressure in solutions rather than gas behavior under compression or expansion. The polytropic process encompasses scenarios ranging from isothermal (\(n=1\)) to adiabatic (\(n=\gamma\)) conditions, making it essential for modeling real gas processes in engineering applications.
Understanding the Isopiestic Line
The isopiestic line represents a curve on a phase diagram where the vapor pressure of a solution remains constant despite changes in temperature or concentration, indicating equilibrium between phases at equal vapor pressures. This line contrasts with the polytropic line, which defines processes with constant polytropic index relating pressure and volume changes in thermodynamic systems. Understanding the isopiestic line is crucial for accurately determining solution properties such as activity coefficients and osmotic pressures, especially in multi-component mixtures where vapor-liquid equilibrium is of interest.
Mathematical Representation of Polytropic and Isopiestic Lines
The polytropic line is mathematically represented by the equation \( PV^n = C \), where \( P \) is pressure, \( V \) is volume, \( n \) is the polytropic index, and \( C \) is a constant specific to the process. The isopiestic line is characterized by constant vapor pressure during the phase change, often modeled with \( P = P_{\text{sat}}(T) \), where \( P_{\text{sat}} \) is the saturation pressure at temperature \( T \). These mathematical representations highlight the distinct thermodynamic processes, with the polytropic equation describing general compressible transformations and the isopiestic equation focusing on equilibrium vapor-liquid conditions.
Key Differences Between Polytropic and Isopiestic Processes
Polytropic lines represent processes where pressure and volume change following the relation \( PV^n = \text{constant} \), encompassing various thermodynamic processes depending on the polytropic index \( n \). Isopiestic lines denote conditions of constant vapor pressure during phase equilibrium in solutions, emphasizing solvent activity and osmotic pressure in mixtures. The key difference lies in polytropic lines describing mechanical work and energy transfer in gases, while isopiestic lines focus on chemical potential and equilibrium in liquid solutions.
Practical Applications of Polytropic Lines
Polytropic lines, characterized by the equation \( PV^n = \text{constant} \), are extensively used in practical engineering applications such as modeling gas compression and expansion processes in turbines, compressors, and nozzles. Unlike isopiestic lines, which deal with constant vapor pressure in thermodynamics for phase equilibrium studies, polytropic lines provide critical insights into energy transfer and work done during non-ideal thermodynamic processes. This makes polytropic analysis essential for optimizing performance and efficiency in various mechanical and chemical engineering systems.
Industrial Relevance of Isopiestic Lines
Isopiestic lines, representing conditions of equal total pressure or chemical potential in industrial processes, are critical for optimizing multi-component phase equilibria and separation techniques, such as distillation and solvent extraction. Unlike polytropic lines that describe pressure-volume-temperature relationships in thermodynamic cycles, isopiestic lines facilitate the design and control of systems where equilibrium partial pressures dictate efficiency and product purity. Leveraging isopiestic data enhances process modeling in petrochemical refining and environmental engineering, leading to improved energy savings and reduced operational costs.
Graphical Representation: Polytropic vs Isopiestic
Polytropic lines on graphs depict processes with varying pressure and volume while maintaining a specific polytropic index, typically displaying curved trajectories dependent on the value of n in the equation PV^n = constant. In contrast, isopiestic lines represent equilibrium states where solvent chemical potential remains constant, often appearing as horizontal or nearly linear lines on concentration versus vapor pressure graphs. The graphical distinction between polytropic and isopiestic lines highlights different thermodynamic properties: polytropic curves demonstrate state changes under energy exchange, whereas isopiestic lines emphasize constant chemical potential in solvent mixtures.
Real-world Examples and Case Studies
Polytropic lines represent processes where gas undergoes changes with a constant polytropic index, commonly observed in turbocharger compressor maps and refrigeration cycles to optimize efficiency. Isopiestic lines illustrate scenarios of equal vapor pressures, crucial in salt basin evaporation studies and geochemical modeling of brine concentrations. Real-world applications in thermodynamics and environmental engineering highlight these lines for predicting system behavior and designing energy-efficient processes.
Summary and Comparative Analysis
Polytropic lines represent processes where pressure and volume changes follow PV^n = constant, allowing the study of thermodynamic transformations with varying heat transfer characteristics. Isopiestic lines depict conditions of equal osmotic pressure, critical in solutions and phase equilibria analysis, reflecting solvent and solute interactions at equilibrium. Comparing both, polytropic lines apply broadly to gaseous states and energy transfer processes, while isopiestic lines specialize in solution chemistry and equilibrium thermodynamics, with distinct applications in engineering and chemical analysis.
Polytropic line Infographic
 libterm.com
libterm.com