Dislocation occurs when bones are forced out of their normal positions in a joint, causing pain, swelling, and immobility. Prompt medical treatment is essential to realign the joint and prevent long-term damage or complications. Explore the rest of this article to understand symptoms, causes, and effective treatments for dislocation.
Table of Comparison
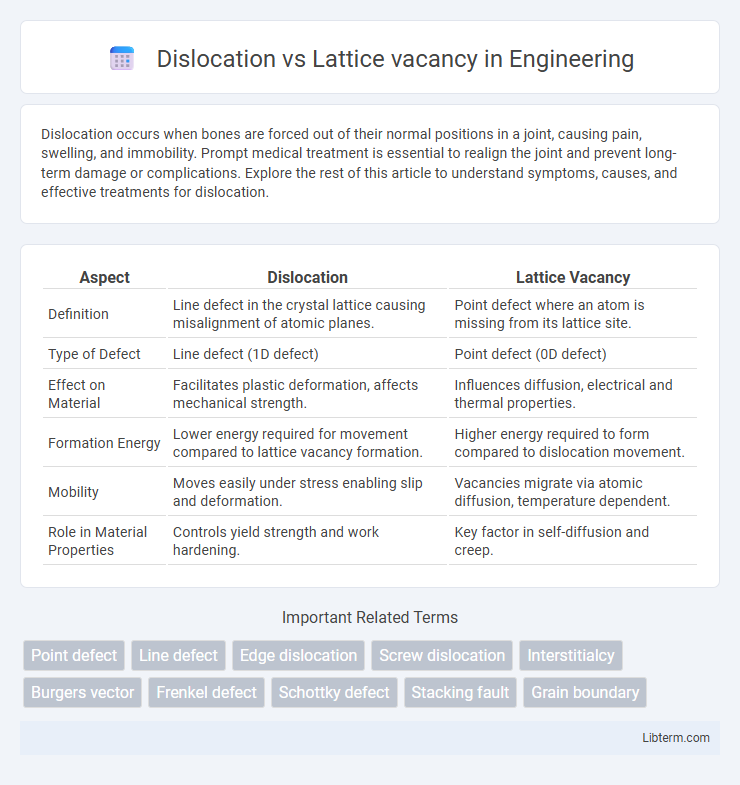
| Aspect | Dislocation | Lattice Vacancy |
|---|---|---|
| Definition | Line defect in the crystal lattice causing misalignment of atomic planes. | Point defect where an atom is missing from its lattice site. |
| Type of Defect | Line defect (1D defect) | Point defect (0D defect) |
| Effect on Material | Facilitates plastic deformation, affects mechanical strength. | Influences diffusion, electrical and thermal properties. |
| Formation Energy | Lower energy required for movement compared to lattice vacancy formation. | Higher energy required to form compared to dislocation movement. |
| Mobility | Moves easily under stress enabling slip and deformation. | Vacancies migrate via atomic diffusion, temperature dependent. |
| Role in Material Properties | Controls yield strength and work hardening. | Key factor in self-diffusion and creep. |
Introduction to Crystal Defects
Dislocations are line defects within a crystal structure characterized by a misalignment of atomic planes, significantly impacting mechanical properties such as yield strength and ductility. Lattice vacancies are point defects where atoms are missing from their regular lattice sites, influencing diffusion rates and electrical conductivity. Both dislocations and vacancies are fundamental crystal defects that alter the physical and chemical behavior of materials.
Defining Dislocation in Crystals
Dislocations in crystals represent line defects where an extra half-plane of atoms is inserted, disrupting the regular atomic arrangement and significantly influencing mechanical properties. Unlike lattice vacancies, which are point defects characterized by missing atoms in the crystal structure, dislocations enable plastic deformation by allowing layers of atoms to slip past each other under stress. The presence and movement of dislocations are critical factors in determining the strength and ductility of crystalline materials.
Understanding Lattice Vacancy
A lattice vacancy is a point defect in a crystal structure where an atom is missing from its regular lattice site, significantly impacting material properties like diffusion and mechanical strength. Unlike dislocations, which are line defects causing slip and plastic deformation, lattice vacancies influence atomic mobility and contribute to phenomena such as self-diffusion and vacancy-mediated diffusion mechanisms in metals and semiconductors. Controlling lattice vacancy concentration is crucial for tailoring thermal conductivity, electrical resistivity, and enhancing material performance in applications ranging from metallurgy to electronic device fabrication.
Types of Dislocations
Dislocations are line defects within a crystal lattice, categorized primarily into edge, screw, and mixed dislocations, each characterized by the relative displacement and orientation of the atomic planes. Edge dislocations involve an extra half-plane of atoms inserted into the lattice, while screw dislocations result from a helical twist of the lattice planes around the dislocation line. Mixed dislocations exhibit properties of both edge and screw types, influencing the mechanical behavior and plastic deformation mechanisms in crystalline materials.
Causes of Lattice Vacancies
Lattice vacancies primarily occur due to thermal vibrations that provide atoms enough energy to leave their lattice sites, creating empty positions within the crystal structure. These vacancies are also caused by rapid cooling or quenching, which traps defects as the lattice contracts faster than atoms can rearrange. Unlike dislocations, which are line defects involving a misalignment of atoms, lattice vacancies are point defects influencing material properties like diffusion and mechanical strength.
Structural Differences: Dislocation vs Lattice Vacancy
Dislocations are line defects within a crystal lattice where atoms are misaligned along a row, causing distortions in the surrounding atomic planes, whereas lattice vacancies are point defects characterized by the absence of an atom at a specific lattice site. Dislocations create plastic deformation by allowing atoms to slip past one another along the slip plane, while lattice vacancies primarily affect diffusion and material density. The structural difference lies in dislocations extending through a line of atoms, contrasting with the localized, zero-dimensional nature of lattice vacancies.
Impact on Material Properties
Dislocations significantly influence the mechanical strength and ductility of materials by facilitating plastic deformation through slip, while lattice vacancies primarily affect diffusion rates and creep behavior at elevated temperatures. The presence of dislocations typically enhances toughness and strain hardening, whereas vacancies contribute to atomic mobility, influencing void formation and material swelling. Understanding the distinct roles of dislocations and vacancies is crucial for tailoring materials with desired mechanical performance and thermal stability.
Detection and Measurement Techniques
Detection and measurement of dislocations primarily utilize techniques like transmission electron microscopy (TEM) and etch pit methods, offering direct visualization of line defects within crystal structures. Lattice vacancies, being point defects, are commonly identified through positron annihilation spectroscopy (PAS) and X-ray diffraction (XRD) analysis, which detect changes in atomic density and local strain fields. Advanced methods such as atom probe tomography (APT) provide atomic-scale resolution for both dislocations and vacancies, enabling precise quantification and spatial mapping.
Role in Material Deformation
Dislocations are line defects that enable plastic deformation in crystalline materials by allowing layers of atoms to slide past each other at lower stress levels compared to perfect crystals. Lattice vacancies, point defects where atoms are missing, primarily facilitate diffusion processes but have a minimal direct role in immediate plastic deformation. The movement and multiplication of dislocations control the strength and ductility of metals, while lattice vacancies influence creep behavior under prolonged stress.
Applications and Industrial Implications
Dislocations enhance metal ductility and strength, vital in manufacturing processes such as forging, welding, and metal forming by allowing controlled plastic deformation. Lattice vacancies influence diffusion rates, critical in semiconductor fabrication, sintering of ceramics, and alloy production, affecting electrical properties and material stability. Understanding the balance between dislocation density and vacancy concentration optimizes mechanical performance and reliability in automotive, aerospace, and electronics industries.
Dislocation Infographic
 libterm.com
libterm.com