Intergranular corrosion occurs along the grain boundaries of metals, where impurities or precipitates cause localized weakening. This type of corrosion can compromise structural integrity without obvious surface damage, making it difficult to detect early. Explore the rest of the article to understand how to identify, prevent, and manage intergranular corrosion effectively.
Table of Comparison
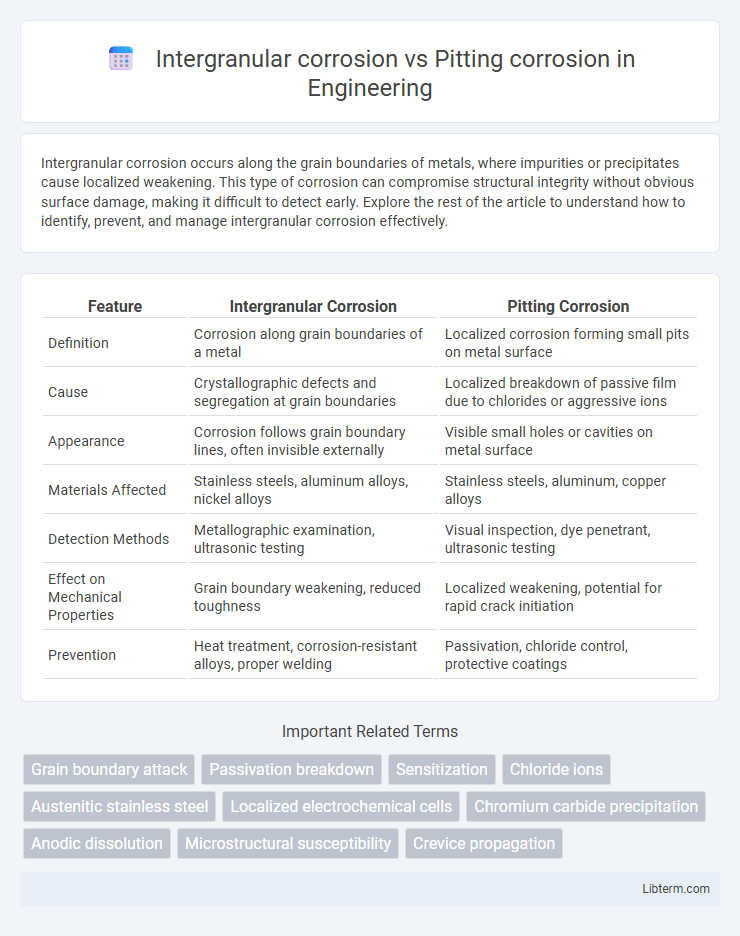
| Feature | Intergranular Corrosion | Pitting Corrosion |
|---|---|---|
| Definition | Corrosion along grain boundaries of a metal | Localized corrosion forming small pits on metal surface |
| Cause | Crystallographic defects and segregation at grain boundaries | Localized breakdown of passive film due to chlorides or aggressive ions |
| Appearance | Corrosion follows grain boundary lines, often invisible externally | Visible small holes or cavities on metal surface |
| Materials Affected | Stainless steels, aluminum alloys, nickel alloys | Stainless steels, aluminum, copper alloys |
| Detection Methods | Metallographic examination, ultrasonic testing | Visual inspection, dye penetrant, ultrasonic testing |
| Effect on Mechanical Properties | Grain boundary weakening, reduced toughness | Localized weakening, potential for rapid crack initiation |
| Prevention | Heat treatment, corrosion-resistant alloys, proper welding | Passivation, chloride control, protective coatings |
Introduction to Intergranular and Pitting Corrosion
Intergranular corrosion targets the grain boundaries of metals, often caused by impurities or precipitates that weaken these areas, leading to structural failure without extensive surface damage. Pitting corrosion creates localized holes or pits, usually on passive metal surfaces such as stainless steel, triggered by chloride ions disrupting the protective oxide layer. Understanding the distinct mechanisms of intergranular and pitting corrosion is vital for predicting metal performance in aggressive environments and selecting appropriate corrosion-resistant materials.
Defining Intergranular Corrosion
Intergranular corrosion is a localized attack occurring along the grain boundaries of a metal, often caused by the precipitation of chromium carbides that deplete chromium in adjacent areas, reducing corrosion resistance. Pitting corrosion, in contrast, manifests as small, deep cavities on the metal surface, typically initiated by chloride ions disrupting the passive film. Understanding the selective nature of intergranular corrosion helps in preventing material failure in stainless steels and welds by controlling heat treatment and alloy composition.
Defining Pitting Corrosion
Pitting corrosion is a localized form of corrosion characterized by the formation of small, deep cavities or pits on the metal surface, often initiated by the breakdown of a passive film. Unlike intergranular corrosion, which progresses along grain boundaries, pitting corrosion penetrates irregularly into the metal, leading to significant structural damage. This corrosion type is commonly observed in stainless steels and aluminum alloys exposed to chloride-containing environments.
Key Differences Between Intergranular and Pitting Corrosion
Intergranular corrosion primarily attacks the grain boundaries in metals, causing weakening along these internal lines, while pitting corrosion results in localized, small holes or pits on the metal surface. Intergranular corrosion often occurs due to sensitization during processes like welding, whereas pitting corrosion is usually initiated by the breakdown of passive oxide layers in chloride-rich environments. The detection of intergranular corrosion requires microscopic examination, but pitting corrosion can often be visually identified due to its characteristic surface pits.
Causes and Contributing Factors
Intergranular corrosion primarily occurs along the grain boundaries of metals due to chromium carbide precipitation that depletes chromium in adjacent areas, weakening corrosion resistance; factors such as improper heat treatment and sensitization contribute significantly. Pitting corrosion arises from localized breakdown of passive oxide layers in metals, often initiated by chloride ions or other aggressive anions that create small, deep cavities; environmental conditions like stagnant water and low oxygen promote its development. Both types are exacerbated by metallurgical flaws and exposure to aggressive chemical environments, but intergranular corrosion targets grain boundaries while pitting attacks discrete surface spots.
Microstructural Mechanisms
Intergranular corrosion occurs along grain boundaries due to the depletion of alloying elements like chromium, which weakens the passive film in stainless steels and results in preferential attack at these boundaries. Pitting corrosion initiates at localized defects or inclusions on the metal surface, where aggressive ions such as chlorides penetrate the passive oxide layer, causing small but deep cavities. The microstructural contrast lies in intergranular corrosion targeting grain boundary precipitates or sensitization effects, while pitting corrosion arises from surface heterogeneities and local breakdown of passivation.
Detection and Identification Methods
Intergranular corrosion detection primarily relies on metallographic examination using optical microscopy after etching with specific reagents such as oxalic acid or the Strauss test to reveal attacks along grain boundaries. Pitting corrosion identification often involves non-destructive techniques like ultrasonic testing or eddy current testing, coupled with visual inspection aided by optical or scanning electron microscopy to analyze pit morphology and depth. Electrochemical methods, including potentiodynamic polarization and electrochemical impedance spectroscopy, serve as complementary tools to distinguish between intergranular and pitting corrosion by characterizing localized attack behavior.
Prevention and Control Strategies
Intergranular corrosion prevention involves proper alloy selection and precise heat treatment processes like solution annealing to restore chromium carbide and prevent grain boundary attack. Pitting corrosion control relies on maintaining passive film integrity through inhibitors, surface treatments, and minimizing chloride exposure. Both corrosion types demand regular inspections and environmental monitoring to detect early signs and apply targeted maintenance strategies.
Real-World Examples and Case Studies
Intergranular corrosion often occurs in stainless steel used in chemical processing plants, where sensitization at grain boundaries leads to material weakening, as seen in failed heat exchanger tubes at petrochemical refineries. Pitting corrosion frequently affects aluminum alloys in marine environments, exemplified by localized damage on ship hulls and offshore structures due to chloride ion penetration. Case studies highlight that intergranular attacks cause large-scale structural degradation, while pitting results in deep, localized perforations risking rapid failure.
Conclusion: Selecting the Right Corrosion Protection
Selecting the right corrosion protection depends on understanding the specific mechanisms of intergranular corrosion and pitting corrosion. Intergranular corrosion targets grain boundaries often due to sensitization in stainless steels, requiring protective measures like proper heat treatment or corrosion-resistant alloys. Pitting corrosion, localized and aggressive, demands coatings or inhibitors that seal microscopic defects to prevent pit initiation and propagation.
Intergranular corrosion Infographic
 libterm.com
libterm.com