Stress corrosion cracking occurs when a material experiences the combined effects of tensile stress and a corrosive environment, leading to unexpected and often sudden failure. Understanding the mechanisms behind this phenomenon is crucial for preventing damage in critical structures such as pipelines, bridges, and aircraft components. Explore the rest of the article to learn how you can identify, mitigate, and manage stress corrosion cracking effectively.
Table of Comparison
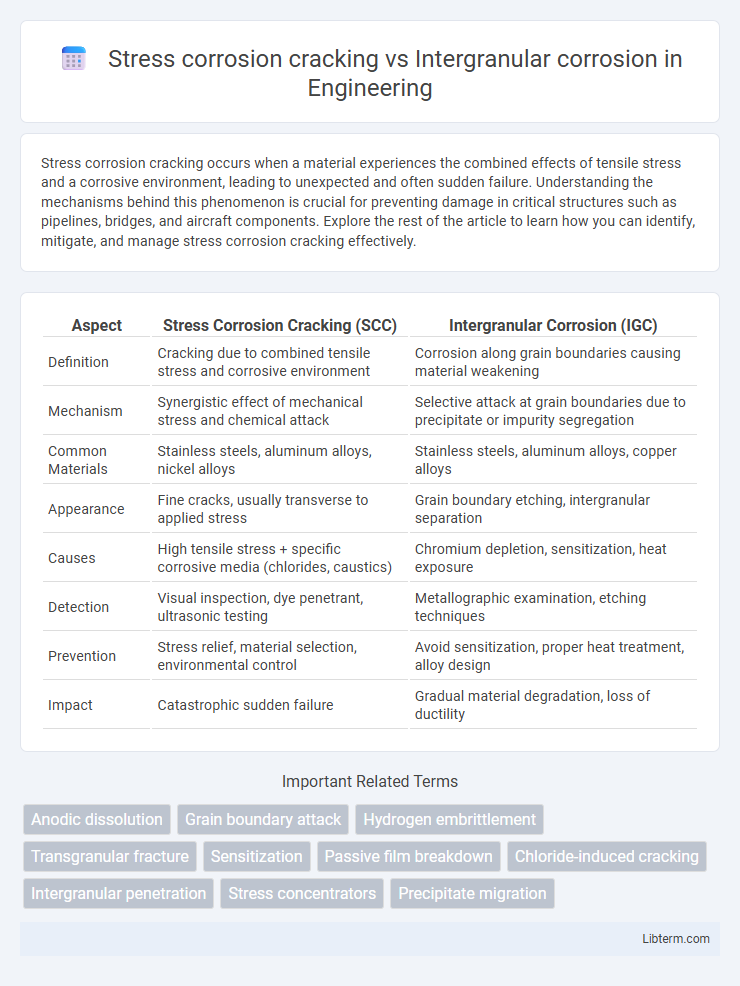
| Aspect | Stress Corrosion Cracking (SCC) | Intergranular Corrosion (IGC) |
|---|---|---|
| Definition | Cracking due to combined tensile stress and corrosive environment | Corrosion along grain boundaries causing material weakening |
| Mechanism | Synergistic effect of mechanical stress and chemical attack | Selective attack at grain boundaries due to precipitate or impurity segregation |
| Common Materials | Stainless steels, aluminum alloys, nickel alloys | Stainless steels, aluminum alloys, copper alloys |
| Appearance | Fine cracks, usually transverse to applied stress | Grain boundary etching, intergranular separation |
| Causes | High tensile stress + specific corrosive media (chlorides, caustics) | Chromium depletion, sensitization, heat exposure |
| Detection | Visual inspection, dye penetrant, ultrasonic testing | Metallographic examination, etching techniques |
| Prevention | Stress relief, material selection, environmental control | Avoid sensitization, proper heat treatment, alloy design |
| Impact | Catastrophic sudden failure | Gradual material degradation, loss of ductility |
Introduction to Corrosion Phenomena
Stress corrosion cracking (SCC) and intergranular corrosion (IGC) are critical forms of localized corrosion that compromise material integrity through distinct mechanisms. SCC occurs due to the combined influence of tensile stress and a corrosive environment, leading to crack initiation and propagation, predominantly in susceptible metals such as stainless steels and aluminum alloys. Intergranular corrosion specifically attacks grain boundaries, often caused by sensitization in alloys like austenitic stainless steel, weakening the microstructure and making materials vulnerable to failure along grain interfaces.
Defining Stress Corrosion Cracking (SCC)
Stress corrosion cracking (SCC) is a failure mechanism characterized by the growth of cracks in a corrosive environment, driven by tensile stress and susceptible material properties. It occurs when a specific combination of tensile stress, corrosive environment, and susceptible alloy leads to brittle cracking along particular crystallographic planes. Unlike intergranular corrosion, which affects grain boundaries, SCC propagates as cracks, significantly compromising the structural integrity of metals such as stainless steel and aluminum alloys.
Understanding Intergranular Corrosion (IGC)
Intergranular corrosion (IGC) specifically attacks the grain boundaries of metals, causing material degradation along these interfaces without significant attack on the grain interiors. This type of corrosion is often triggered by sensitization processes that lead to precipitation of chromium carbides at grain boundaries in stainless steels, reducing corrosion resistance locally. Unlike stress corrosion cracking, which involves crack propagation under tensile stress and corrosive environments, IGC primarily results from chemical or electrochemical attacks localized at grain boundaries, leading to material weakening and potential structural failure.
Mechanisms of Stress Corrosion Cracking
Stress corrosion cracking (SCC) occurs due to the combined effects of tensile stress and a corrosive environment, causing crack initiation and propagation along susceptible microstructural paths in metals. The mechanism involves anodic dissolution or hydrogen embrittlement at crack tips, influenced by factors such as material composition, residual stresses, and environmental conditions like chloride presence or pH levels. In contrast, intergranular corrosion (IGC) primarily results from grain boundary sensitization and precipitate formation, leading to preferential attack along grain boundaries without the requirement of tensile stress.
Mechanisms of Intergranular Corrosion
Intergranular corrosion occurs along the grain boundaries of a metal, primarily due to the precipitation of chromium carbides that deplete chromium in these regions, reducing corrosion resistance. This selective attack weakens the grain boundaries, causing material failure without significant attack on the grain interiors. Stress corrosion cracking, unlike intergranular corrosion, involves the combined action of tensile stress and a corrosive environment, leading to the formation and propagation of cracks, often following specific crystallographic paths.
Key Differences Between SCC and IGC
Stress corrosion cracking (SCC) involves the growth of cracks in a corrosive environment under tensile stress, typically propagating through the grain interiors. Intergranular corrosion (IGC) primarily attacks the grain boundaries due to localized chemical changes, often without the presence of significant tensile stress. SCC is characterized by brittle fracture with crack propagation under stress, while IGC leads to grain boundary weakening and material embrittlement without necessarily forming large cracks.
Factors Influencing SCC and IGC
Stress corrosion cracking (SCC) is influenced by tensile stress, corrosive environment, and material susceptibility, often occurring in metals exposed to specific chemical agents like chlorides or hydrogen sulfide. Intergranular corrosion (IGC) primarily depends on grain boundary chemistry, sensitization due to heat treatment, and environmental factors such as temperature and pH. Both SCC and IGC are exacerbated by microstructural changes, including precipitation of secondary phases and depletion of protective elements at grain boundaries.
Materials Susceptible to SCC and IGC
Stress corrosion cracking (SCC) predominantly affects high-strength alloys such as austenitic stainless steels, high-strength aluminum alloys, and certain nickel-based superalloys, where tensile stress and corrosive environments interact to initiate crack propagation. Intergranular corrosion (IGC) mainly targets materials with sensitized grain boundaries, especially austenitic stainless steels that have been exposed to temperatures causing chromium carbide precipitation, as well as certain aluminum alloys and brass. Understanding the microstructural susceptibility of alloys, including grain boundary chemistry and stress conditions, is crucial for preventing both SCC and IGC in critical engineering applications.
Prevention and Mitigation Strategies
Stress corrosion cracking (SCC) prevention focuses on controlling tensile stress, reducing corrosive environments, and using resistant alloys such as stainless steel and nickel alloys. Intergranular corrosion (IGC) mitigation emphasizes grain boundary stabilization through heat treatments like solution annealing and avoiding sensitization by minimizing exposure to temperatures that promote chromium carbide precipitation. Both phenomena benefit from protective coatings, regular inspection, and cathodic protection to enhance material durability in aggressive environments.
Case Studies: Industrial Implications
Stress corrosion cracking (SCC) in industrial settings often involves high-tensile materials exposed to corrosive environments, as seen in petrochemical pipelines where chloride-induced SCC led to catastrophic failures. Intergranular corrosion (IGC) frequently affects stainless steels in power plants, with documented cases of sensitized grain boundaries causing significant degradation in heat exchangers. Both phenomena demand targeted monitoring and material selection strategies to mitigate costly downtime and ensure structural integrity in critical infrastructures.
Stress corrosion cracking Infographic
 libterm.com
libterm.com