Suspension systems play a crucial role in ensuring vehicle stability, comfort, and control by absorbing shocks from road irregularities. Proper maintenance of your suspension components enhances safety and prolongs the lifespan of your vehicle. Explore the rest of this article to learn how to identify suspension issues and maintain optimal performance.
Table of Comparison
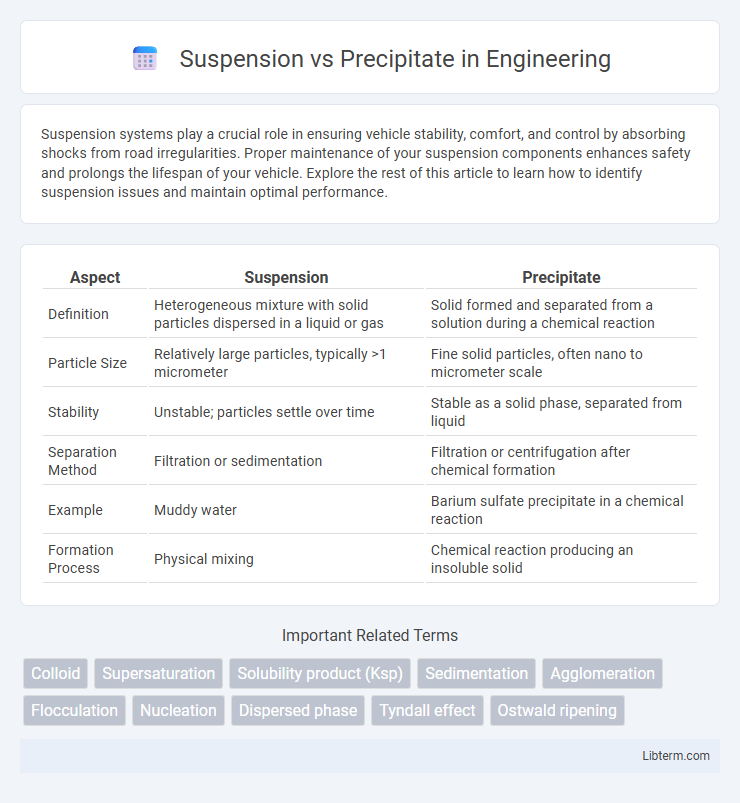
| Aspect | Suspension | Precipitate |
|---|---|---|
| Definition | Heterogeneous mixture with solid particles dispersed in a liquid or gas | Solid formed and separated from a solution during a chemical reaction |
| Particle Size | Relatively large particles, typically >1 micrometer | Fine solid particles, often nano to micrometer scale |
| Stability | Unstable; particles settle over time | Stable as a solid phase, separated from liquid |
| Separation Method | Filtration or sedimentation | Filtration or centrifugation after chemical formation |
| Example | Muddy water | Barium sulfate precipitate in a chemical reaction |
| Formation Process | Physical mixing | Chemical reaction producing an insoluble solid |
Introduction to Suspension and Precipitate
Suspensions are heterogeneous mixtures where solid particles are dispersed throughout a liquid but remain undissolved, creating a cloudy appearance due to particle size typically greater than 1 micrometer. Precipitates form when a solid emerges from a chemical reaction in a solution, causing dissolved ions to combine and settle out of the liquid phase. Both phenomena involve solid-liquid interactions, but suspensions involve physical dispersion, while precipitates result from chemical changes producing an insoluble solid.
Defining Suspension: Key Characteristics
A suspension is a heterogeneous mixture where solid particles are dispersed throughout a liquid or gas but are not dissolved, causing the particles to remain visible and settle over time. Key characteristics include particle sizes larger than 1 micrometer, the ability to separate by filtration, and the presence of a cloudy or murky appearance. Unlike precipitates, suspended particles in a suspension remain mixed temporarily with agitation but eventually settle due to gravity.
Understanding Precipitate: Main Features
Precipitate is a solid that forms in a solution during a chemical reaction, typically resulting from the combination of two soluble salts. It appears as small particles that settle at the bottom of the container, differentiating it from a suspension where particles remain dispersed. Key features include insolubility in the solvent, often identifiable by changes in color or cloudiness, and its ability to be separated through filtration.
Formation Processes: Suspension vs Precipitate
Suspensions form when solid particles are dispersed throughout a liquid or gas without dissolving, typically resulting from mechanical agitation or mixing. Precipitates develop through chemical reactions that cause dissolved substances to become insoluble and settle out of the solution. The formation process of suspensions involves physical dispersion, while precipitates result from changes in solubility and chemical composition.
Visual Differences and Appearance
Suspensions appear cloudy or murky due to the larger particles dispersed throughout the liquid, often settling over time and creating a distinct layer between the solid and liquid phases. Precipitates form as solid particles that settle out from a solution and typically accumulate as distinct, visible solids at the bottom of the container. Visually, suspensions remain mixed and turbid before settling, while precipitates are solid masses that clearly separate from the liquid.
Particle Size and Distribution
Suspension consists of larger, often visible particles typically exceeding 1 micrometer, leading to heterogeneous distribution where particles may settle over time. Precipitates form from chemical reactions, resulting in fine particles usually below 1 micrometer that aggregate to create a solid mass with a relatively uniform particle size distribution. The particle size in suspensions affects stability and sedimentation rate, whereas in precipitates, particle size influences purity and filtration efficiency.
Stability and Settling Behavior
Suspensions consist of larger particles dispersed in a liquid that remain stable for a limited time but eventually settle due to gravity, forming a distinguishable layer. Precipitates form when solutes chemically react and produce solid particles that rapidly aggregate, resulting in faster and more permanent settling. The stability of suspensions relies on particle size and density, while precipitates exhibit stronger intermolecular forces, leading to quicker sedimentation and less stability in the dispersion.
Common Examples in Everyday Life
Suspension often occurs in muddy water, where soil particles remain dispersed but eventually settle, while precipitate forms in chemical reactions like mixing silver nitrate and sodium chloride, producing solid silver chloride. Common suspensions include flour in water or sand in water, where particles are visible and separate over time. Precipitates frequently appear in hard water scale buildup and many kitchen cooking reactions, such as curd forming when lemon juice is added to milk.
Analytical Methods for Differentiation
Suspensions and precipitates can be differentiated using analytical methods such as microscopic examination, where suspensions show larger, dispersed particles while precipitates form solid aggregates that settle out of solution. Spectroscopic techniques like UV-Vis or turbidity measurements quantify particle size and concentration differences, with suspensions exhibiting higher turbidity levels. Centrifugation also aids in separation and analysis, as precipitates form a compact pellet whereas suspensions yield dispersed particles in the supernatant.
Practical Applications and Implications
Suspensions, composed of larger particles dispersed in a fluid, are commonly used in pharmaceuticals for drug delivery systems that require slow release and easy suspension mixing. Precipitates, formed by chemical reactions resulting in solid particles, play a critical role in water treatment for removing contaminants and heavy metals through selective precipitation. Understanding the differences between suspensions and precipitates enables optimization in industrial processes such as wastewater treatment, material synthesis, and pharmaceutical formulation.
Suspension Infographic
 libterm.com
libterm.com