Multiple myeloma is a type of blood cancer that affects plasma cells in the bone marrow, leading to bone damage, anemia, and infections. Early diagnosis and treatment are crucial to managing symptoms and improving quality of life. Explore this article to understand the latest advances in multiple myeloma diagnosis and treatment options.
Table of Comparison
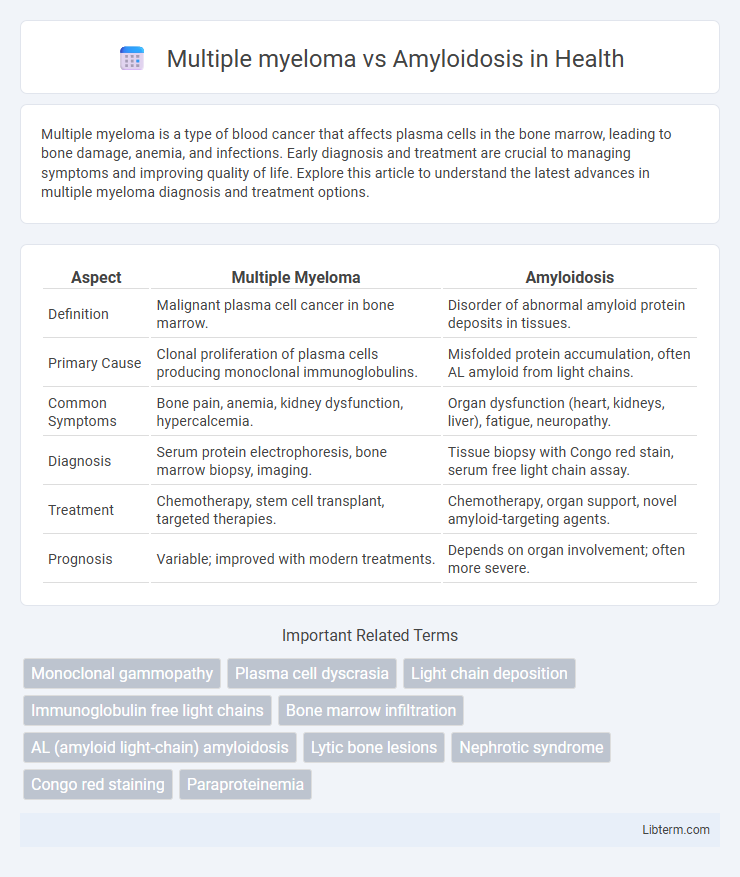
| Aspect | Multiple Myeloma | Amyloidosis |
|---|---|---|
| Definition | Malignant plasma cell cancer in bone marrow. | Disorder of abnormal amyloid protein deposits in tissues. |
| Primary Cause | Clonal proliferation of plasma cells producing monoclonal immunoglobulins. | Misfolded protein accumulation, often AL amyloid from light chains. |
| Common Symptoms | Bone pain, anemia, kidney dysfunction, hypercalcemia. | Organ dysfunction (heart, kidneys, liver), fatigue, neuropathy. |
| Diagnosis | Serum protein electrophoresis, bone marrow biopsy, imaging. | Tissue biopsy with Congo red stain, serum free light chain assay. |
| Treatment | Chemotherapy, stem cell transplant, targeted therapies. | Chemotherapy, organ support, novel amyloid-targeting agents. |
| Prognosis | Variable; improved with modern treatments. | Depends on organ involvement; often more severe. |
Introduction: Understanding Multiple Myeloma and Amyloidosis
Multiple myeloma is a malignant plasma cell disorder characterized by clonal proliferation within the bone marrow, leading to bone lesions, anemia, and renal dysfunction. Amyloidosis involves the abnormal deposition of misfolded amyloid proteins in tissues, causing organ dysfunction; it often coexists with plasma cell disorders like multiple myeloma. Differentiating these conditions is critical due to overlapping symptoms but distinct pathological mechanisms and treatment strategies.
Definitions and Disease Overview
Multiple myeloma is a malignant plasma cell disorder characterized by the clonal proliferation of plasma cells in the bone marrow, leading to bone lesions, anemia, hypercalcemia, and renal impairment. Amyloidosis is a group of diseases caused by extracellular deposition of misfolded amyloid proteins, which disrupt normal tissue structure and function, commonly affecting the heart, kidneys, and nerves. While multiple myeloma involves malignant plasma cell proliferation and monoclonal protein production, amyloidosis primarily results from amyloid fibril accumulation due to protein misfolding, sometimes associated with plasma cell dyscrasias in AL (light-chain) amyloidosis.
Causes and Risk Factors
Multiple myeloma is caused by the uncontrolled proliferation of malignant plasma cells in the bone marrow, often linked to genetic mutations and environmental exposures like radiation. Amyloidosis results from the abnormal accumulation of amyloid proteins in tissues, with risk factors including chronic inflammatory diseases, multiple myeloma itself, and hereditary mutations in amyloid precursor proteins. Both diseases share overlapping risk factors such as age over 60 and male gender, but their distinct pathological mechanisms differentiate their development.
Pathophysiology: How the Diseases Develop
Multiple myeloma develops from malignant plasma cell proliferation in the bone marrow, leading to excessive production of abnormal monoclonal immunoglobulins or light chains. Amyloidosis occurs when misfolded protein fragments, including light chains derived from plasma cells, aggregate into insoluble amyloid fibrils that deposit extracellularly in tissues and organs. The pathogenic link involves monoclonal light chains causing tissue damage in amyloidosis, contrasting with bone marrow infiltration and skeletal destruction driving multiple myeloma progression.
Key Differences in Symptoms
Multiple myeloma primarily causes bone pain, anemia, frequent infections, and hypercalcemia due to malignant plasma cells accumulating in the bone marrow. Amyloidosis symptoms vary depending on organ involvement, commonly including kidney dysfunction, heart failure, neuropathy, and characteristic protein deposits disrupting tissue function. While multiple myeloma targets skeletal health and blood abnormalities, amyloidosis manifests through systemic organ damage caused by amyloid protein accumulation.
Diagnostic Approaches and Biomarkers
Multiple myeloma diagnosis relies on serum protein electrophoresis (SPEP), immunofixation, and measurement of free light chains to detect monoclonal plasma cell proliferation. Amyloidosis diagnosis emphasizes tissue biopsy with Congo red staining and mass spectrometry to identify amyloid fibrils, alongside biomarker assessment such as NT-proBNP and cardiac troponins for organ involvement. Both diseases require bone marrow biopsy, but amyloidosis detection often involves fat pad aspirate or involved organ biopsy to confirm amyloid deposits.
Treatment Options and Management Strategies
Multiple myeloma treatment primarily includes targeted therapies such as proteasome inhibitors (bortezomib), immunomodulatory drugs (lenalidomide), and autologous stem cell transplantation, focusing on reducing malignant plasma cells and managing bone damage. Amyloidosis management involves chemotherapy regimens targeting the underlying plasma cell disorder, often with melphalan and dexamethasone, alongside supportive care to address organ dysfunction caused by amyloid deposits. Both diseases require careful monitoring for treatment-related complications, with emphasis on personalized therapy plans to improve patient outcomes and quality of life.
Prognosis and Survival Rates
Multiple myeloma has a median survival rate of approximately 5 to 7 years with current therapies, while prognosis significantly varies based on stage and response to treatment. Amyloidosis, particularly light-chain (AL) amyloidosis, presents a more variable prognosis, often with a median survival of less than 3 years if untreated, but improved outcomes are seen with early diagnosis and modern chemotherapy protocols. Both conditions require timely intervention, with survival rates heavily influenced by organ involvement and the effectiveness of targeted treatments.
Complications and Long-Term Effects
Multiple myeloma often leads to complications such as bone fractures, kidney damage, anemia, and hypercalcemia, with long-term effects including persistent bone pain and increased infection risk. Amyloidosis primarily causes organ dysfunction, particularly in the heart, kidneys, and nervous system, resulting in heart failure, nephrotic syndrome, and peripheral neuropathy over time. Both diseases significantly impact patient quality of life but differ markedly in organ involvement and progression patterns.
Emerging Research and Future Therapies
Emerging research in Multiple Myeloma centers on targeted therapies such as bispecific antibodies and CAR-T cell treatments, showing promise in improving remission rates. For Amyloidosis, novel approaches include RNA interference and monoclonal antibodies aimed at reducing amyloid deposits, significantly advancing disease management. Future therapies are increasingly personalized, leveraging genetic and molecular profiling to optimize efficacy and minimize adverse effects in both conditions.
Multiple myeloma Infographic
 libterm.com
libterm.com