Viral load measures the amount of virus present in a person's body, playing a crucial role in diagnosing and monitoring infections like HIV and COVID-19. Understanding your viral load helps assess disease progression and the effectiveness of treatment. Explore the rest of this article to learn how viral load impacts your health management.
Table of Comparison
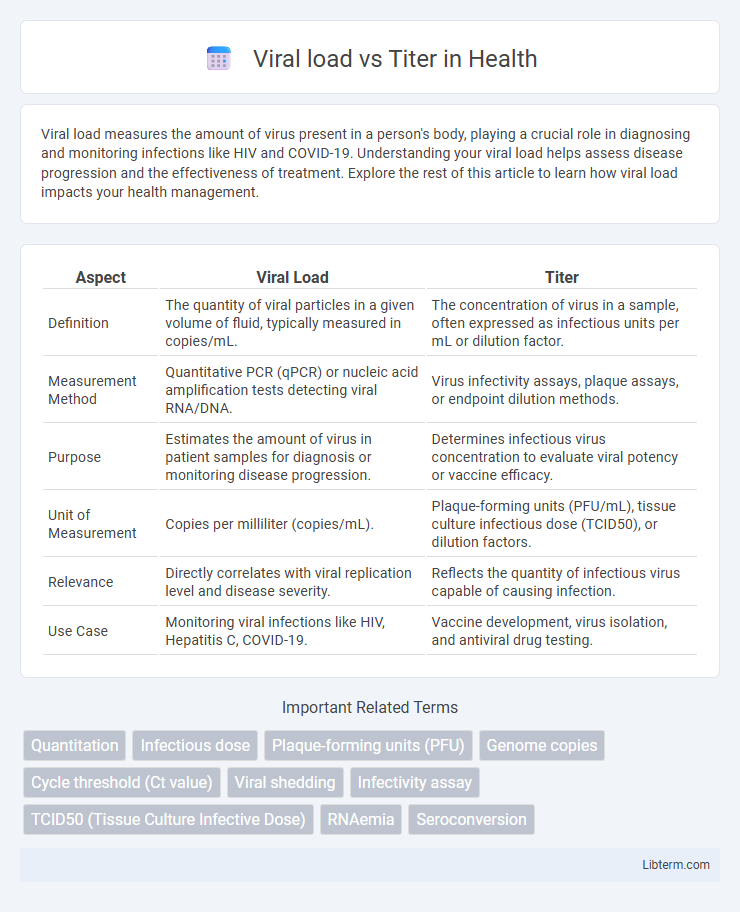
| Aspect | Viral Load | Titer |
|---|---|---|
| Definition | The quantity of viral particles in a given volume of fluid, typically measured in copies/mL. | The concentration of virus in a sample, often expressed as infectious units per mL or dilution factor. |
| Measurement Method | Quantitative PCR (qPCR) or nucleic acid amplification tests detecting viral RNA/DNA. | Virus infectivity assays, plaque assays, or endpoint dilution methods. |
| Purpose | Estimates the amount of virus in patient samples for diagnosis or monitoring disease progression. | Determines infectious virus concentration to evaluate viral potency or vaccine efficacy. |
| Unit of Measurement | Copies per milliliter (copies/mL). | Plaque-forming units (PFU/mL), tissue culture infectious dose (TCID50), or dilution factors. |
| Relevance | Directly correlates with viral replication level and disease severity. | Reflects the quantity of infectious virus capable of causing infection. |
| Use Case | Monitoring viral infections like HIV, Hepatitis C, COVID-19. | Vaccine development, virus isolation, and antiviral drug testing. |
Understanding Viral Load: Definition and Importance
Viral load refers to the quantity of virus present in a specific volume of body fluid, commonly measured using techniques like quantitative PCR to assess infection severity and monitor treatment efficacy. Understanding viral load is crucial for managing infectious diseases such as HIV, hepatitis, and COVID-19, as it provides insight into viral replication dynamics and patient prognosis. Unlike viral titer, which measures infectious virus particles capable of forming plaques or causing cytopathic effects, viral load quantifies total viral genetic material, offering a direct metric for viral burden.
What is Viral Titer? Key Concepts Explained
Viral titer refers to the concentration of virus particles in a given volume of fluid, measured through assays like plaque assays or TCID50, indicating infectious virus quantity. Unlike viral load, which quantifies total viral genetic material by methods such as quantitative PCR, viral titer assesses the number of viable, infectious virions. Understanding viral titer is crucial for evaluating virus infectivity, vaccine efficacy, and antiviral drug performance in virology research and clinical diagnostics.
Viral Load vs Titer: Core Differences
Viral load quantifies the total amount of viral RNA or DNA in a sample, providing a direct measure of infection severity, while viral titer indicates the concentration of infectious viral particles capable of causing infection in host cells. Viral load is typically measured using quantitative PCR (qPCR) techniques, offering precise nucleic acid quantification, whereas viral titer relies on culture-based assays such as plaque or TCID50 assays to determine infectivity. Understanding these core differences is crucial for diagnostic accuracy, treatment monitoring, and epidemiological studies.
Measurement Techniques for Viral Load and Titer
Measurement techniques for viral load primarily involve quantitative PCR (qPCR) and reverse transcription PCR (RT-PCR), which detect and quantify viral RNA or DNA in clinical samples with high sensitivity and specificity. In contrast, viral titer is commonly measured using plaque assays, tissue culture infectious dose 50% (TCID50) assays, and endpoint dilution assays that evaluate the number of infectious viral particles in a sample. While viral load assays provide rapid and precise quantification of viral genomes, titer assays assess infectivity, making both essential for comprehensive viral analysis.
Clinical Significance of Viral Load
Viral load quantifies the amount of viral RNA or DNA in a patient's blood, serving as a critical indicator for disease progression and treatment efficacy, especially in infections like HIV and hepatitis. Unlike viral titer, which measures the quantity of infectious virus particles, viral load provides a more precise assessment of replication activity and risk of transmission. Clinically, monitoring viral load guides therapeutic decisions, predicts patient outcomes, and assists in evaluating antiretroviral therapy success.
Diagnostic Applications of Viral Titer
Viral titer quantifies the concentration of infectious viral particles in a sample, making it critical for diagnostic applications such as determining infection severity and monitoring treatment efficacy. Diagnostic viral titer assays, including plaque assays and TCID50, provide precise measures of viable virus, which is essential for clinical decision-making and epidemiological studies. Unlike viral load tests that often quantify viral genetic material, viral titer results directly reflect infectivity and viral replication capability.
Interpreting Results: Viral Load and Titer in Patient Care
Viral load quantifies the total amount of viral RNA or DNA in a patient's blood, serving as a critical marker for infection severity and treatment efficacy. Titer measures the concentration of antibodies or virus particles capable of inducing an immune response, indicating exposure level or immunity status. Accurate interpretation of viral load and titer results guides clinicians in tailoring patient care by monitoring disease progression and adjusting therapeutic strategies.
Factors Influencing Viral Load and Titer Values
Viral load and titer values are influenced by various factors including the stage of infection, immune response, and viral replication efficiency. Host factors such as age, genetics, and co-infections impact viral load, while assay sensitivity and sample type critically affect titer measurements. Environmental conditions and treatment interventions also play significant roles in modulating both viral load and titer results.
Limitations and Challenges in Measurement
Viral load measurement quantifies the amount of viral RNA or DNA in a sample but faces challenges such as variability in sample collection, differences in assay sensitivity, and the influence of viral replication dynamics. Titer measurement, often based on infectivity or antibody binding assays, is limited by the requirement for viable virus or specific immune responses, introducing variability due to host factors and assay conditions. Both methods encounter difficulties in standardization, reproducibility, and interpretation, particularly in clinical and environmental contexts where virus heterogeneity and sample complexity affect accuracy.
Future Perspectives: Viral Load and Titer in Modern Medicine
Future perspectives in modern medicine emphasize the integration of viral load and titer measurements to enhance precision in diagnosing, monitoring, and treating viral infections. Advanced molecular techniques and digital diagnostics are poised to improve the accuracy and speed of viral quantification, enabling personalized therapeutic strategies and real-time tracking of disease progression. Innovations in viral load and titer analysis will drive the development of targeted antiviral therapies and vaccine efficacy assessments, shaping the future of infectious disease management.
Viral load Infographic
 libterm.com
libterm.com