Asymmetric designs create visual interest by intentionally avoiding symmetry, offering unique and dynamic aesthetics that capture attention. They provide balance through contrast, making compositions more engaging and memorable. Explore the rest of the article to discover how asymmetric elements can enhance your creative projects.
Table of Comparison
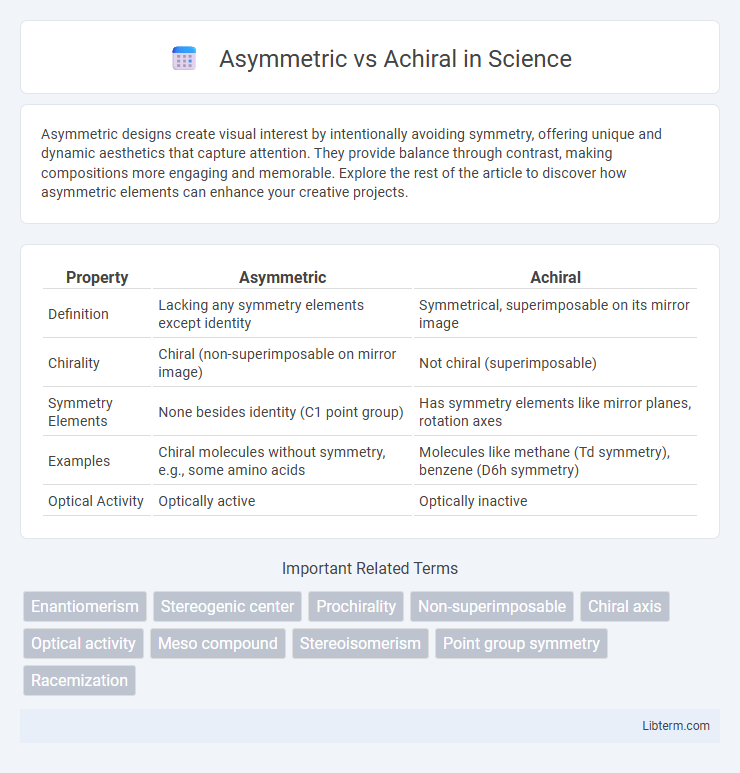
| Property | Asymmetric | Achiral |
|---|---|---|
| Definition | Lacking any symmetry elements except identity | Symmetrical, superimposable on its mirror image |
| Chirality | Chiral (non-superimposable on mirror image) | Not chiral (superimposable) |
| Symmetry Elements | None besides identity (C1 point group) | Has symmetry elements like mirror planes, rotation axes |
| Examples | Chiral molecules without symmetry, e.g., some amino acids | Molecules like methane (Td symmetry), benzene (D6h symmetry) |
| Optical Activity | Optically active | Optically inactive |
Introduction to Molecular Symmetry
Molecular symmetry classifies molecules based on their spatial arrangement, distinguishing asymmetric and achiral structures. Asymmetric molecules lack symmetry elements such as mirror planes or centers of inversion, resulting in non-superimposable mirror images and often exhibiting chirality. Achiral molecules possess symmetry elements that make them superimposable on their mirror images, leading to identical enantiomers and no optical activity.
Defining Asymmetric and Achiral Molecules
Asymmetric molecules contain at least one chiral center, typically a carbon atom bonded to four distinct substituents, resulting in non-superimposable mirror images called enantiomers. Achiral molecules lack chiral centers or possess internal planes of symmetry, making them superimposable on their mirror images. The presence or absence of chirality significantly influences the molecule's optical activity and stereochemical properties.
Key Differences Between Asymmetric and Achiral Compounds
Asymmetric compounds contain at least one chiral center, leading to non-superimposable mirror images and optical activity, whereas achiral compounds lack chiral centers or have symmetrical arrangements that prevent optical isomerism. The presence of an asymmetric carbon atom in molecules like amino acids results in stereoisomers with distinct spatial configurations, while achiral molecules such as methane exhibit identical mirror images. This fundamental difference affects properties like optical rotation, biological activity, and chemical reactivity.
Importance of Chirality in Chemistry
Chirality plays a crucial role in chemistry, impacting molecular interactions and biological activity due to the distinct spatial arrangements of asymmetric molecules versus achiral ones. Asymmetric molecules possess non-superimposable mirror images, giving rise to enantiomers with unique chemical and pharmacological properties, while achiral molecules lack this stereochemical diversity. Understanding the differences in chirality is essential for drug design, catalytic processes, and materials science, where the stereochemistry directly influences function and efficacy.
Real-World Examples of Asymmetric Molecules
Asymmetric molecules, characterized by having chiral centers, include real-world examples like amino acids such as L-alanine and pharmaceuticals like thalidomide, which exhibit optical activity critical for biological function and drug efficacy. Achiral molecules lack chiral centers and mirror image asymmetry, seen in compounds like benzene and chloroform, which do not rotate plane-polarized light. The distinction between asymmetric (chiral) and achiral molecules significantly impacts stereochemistry applications in drug design, synthetic chemistry, and molecular recognition processes.
Real-World Examples of Achiral Molecules
Achiral molecules lack chirality and are superimposable on their mirror images, unlike asymmetric molecules that have non-superimposable mirror images. Real-world examples of achiral molecules include carbon dioxide (CO2), benzene (C6H6), and ethane (C2H6), which exhibit symmetrical structures preventing chirality. Achirality in these compounds influences their chemical behavior, such as benzene's planar symmetry contributing to its aromatic stability.
Methods for Identifying Molecular Symmetry
Methods for identifying molecular symmetry rely heavily on analyzing the presence or absence of symmetry elements such as mirror planes, rotational axes, and inversion centers. Asymmetric molecules lack any of these symmetry elements, making techniques like X-ray crystallography and NMR spectroscopy essential for confirming chirality and stereochemical configurations. Achiral molecules, possessing symmetry elements like a plane of symmetry or a center of inversion, can be identified using group theory and computational chemistry tools that calculate point groups and molecular orbitals.
Applications in Pharmaceuticals and Material Science
Asymmetric molecules play a crucial role in pharmaceuticals by enabling the production of enantiomerically pure drugs, which often exhibit enhanced therapeutic effects and reduced side effects compared to their racemic mixtures, while achiral compounds are preferred in material science for their uniform physical properties and stability in polymer synthesis and nanomaterials. The chirality of asymmetric molecules influences drug-receptor interactions and metabolic pathways, essential for designing selective and potent pharmaceuticals. In contrast, achiral substances facilitate the creation of isotropic materials and consistent performance in applications such as coatings, adhesives, and electronic devices.
Common Misconceptions About Asymmetric and Achiral
Many confuse asymmetric and achiral molecules, assuming all asymmetric molecules are chiral, but asymmetry alone does not guarantee chirality, as some asymmetric molecules can be achiral due to internal planes of symmetry. Achiral molecules lack handedness or optical activity despite structural complexity, while asymmetric molecules specifically have no symmetry elements that generate superimposable mirror images. Misunderstandings often arise from conflating asymmetry with chirality, overlooking that asymmetric centers can exist in both chiral and achiral contexts.
Summary and Future Perspectives
Asymmetric molecules possess chirality due to the presence of non-superimposable mirror images, playing a crucial role in pharmaceuticals and materials science by enabling selective interactions. Achiral molecules lack such stereoisomerism, exhibiting symmetry that influences their physical and chemical properties differently. Future research aims to exploit asymmetric synthesis for more efficient drug development and explore achiral compounds in novel applications like catalysis and optoelectronics.
Asymmetric Infographic
 libterm.com
libterm.com