Electrolytes are essential minerals that conduct electrical impulses in the body, regulating hydration, nerve function, and muscle contractions. Maintaining proper electrolyte balance is crucial for optimal physical performance and preventing issues like dehydration and muscle cramps. Explore this article to understand how electrolytes impact your health and ways to keep them balanced effectively.
Table of Comparison
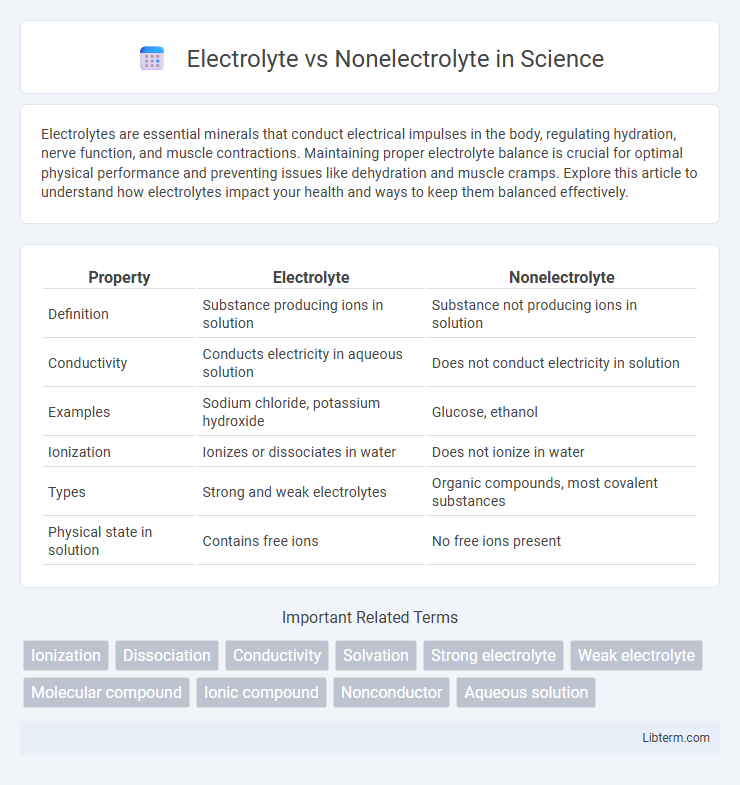
| Property | Electrolyte | Nonelectrolyte |
|---|---|---|
| Definition | Substance producing ions in solution | Substance not producing ions in solution |
| Conductivity | Conducts electricity in aqueous solution | Does not conduct electricity in solution |
| Examples | Sodium chloride, potassium hydroxide | Glucose, ethanol |
| Ionization | Ionizes or dissociates in water | Does not ionize in water |
| Types | Strong and weak electrolytes | Organic compounds, most covalent substances |
| Physical state in solution | Contains free ions | No free ions present |
Introduction to Electrolytes and Nonelectrolytes
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electricity, such as sodium chloride (NaCl) and potassium chloride (KCl). Nonelectrolytes, like glucose and urea, dissolve in water but do not produce ions, thus they do not conduct electricity. Understanding the distinction between electrolytes and nonelectrolytes is essential in fields like chemistry, biology, and medicine, where ion balance and electrical conductivity impact cellular functions and industrial processes.
Defining Electrolytes: Key Characteristics
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electrical current. Key characteristics include ionic dissociation, presence of charged particles (cations and anions), and electrical conductivity in aqueous solutions. Nonelectrolytes, in contrast, do not dissociate into ions and thus do not facilitate electrical conduction in solution.
Defining Nonelectrolytes: Key Characteristics
Nonelectrolytes are substances that do not dissociate into ions when dissolved in water, resulting in solutions that do not conduct electricity. Common nonelectrolytes include glucose, urea, and alcohols, which dissolve as intact molecules without producing charged particles. Their molecular structure and lack of ionic bonds distinguish them from electrolytes, making nonelectrolytes essential in applications requiring non-conductive solutions.
Types of Electrolytes: Strong vs. Weak
Strong electrolytes completely dissociate into ions in aqueous solutions, resulting in high electrical conductivity, examples include sodium chloride and hydrochloric acid. Weak electrolytes partially dissociate, such as acetic acid and ammonia, producing fewer ions and lower conductivity. This difference impacts their behavior in chemical reactions and their use in applications like batteries and biological systems.
Common Examples of Electrolytes
Common examples of electrolytes include sodium chloride (table salt), potassium chloride, calcium chloride, and magnesium sulfate, all of which dissociate into ions in aqueous solutions, enhancing electrical conductivity. These substances are vital in biological systems for nerve signal transmission and muscle function due to their ionic properties. In contrast, nonelectrolytes like sugar and ethanol do not ionize in water and therefore do not conduct electricity.
Common Examples of Nonelectrolytes
Common examples of nonelectrolytes include sugar (sucrose), ethanol, and urea, which dissolve in water without producing ions. Unlike electrolytes such as sodium chloride or potassium nitrate that conduct electricity due to ionization, nonelectrolytes do not dissociate in aqueous solutions. These substances retain their molecular form, resulting in solutions that are electrically non-conductive and important in applications requiring non-conductive solvents.
Electrical Conductivity in Solutions
Electrolytes dissociate into ions when dissolved in water, resulting in high electrical conductivity due to the free movement of charged particles. Nonelectrolytes, such as sugar or ethanol, do not ionize in solution, resulting in negligible or no electrical conductivity. The degree of ionization directly influences the conductivity levels, making electrolytes crucial for processes requiring efficient charge transport in solutions.
Real-World Applications of Electrolytes
Electrolytes, such as sodium chloride and potassium chloride, are essential in real-world applications like medical treatments, where they regulate nerve and muscle function through ion conduction in bodily fluids. Unlike nonelectrolytes, which do not dissociate into ions, electrolytes enable vital processes in batteries, fuel cells, and water purification by facilitating electrical conductivity. The use of electrolytes in sports drinks also restores fluid balance and prevents dehydration by replenishing key ions lost during exercise.
Biological Importance of Electrolytes vs. Nonelectrolytes
Electrolytes such as sodium, potassium, and calcium ions are crucial for maintaining nerve impulse transmission, muscle contraction, and cellular hydration in biological systems. Nonelectrolytes like glucose and urea, while essential for energy metabolism and waste removal, do not dissociate into ions and therefore lack direct involvement in electrical signaling and osmotic balance. The ionic nature of electrolytes supports critical physiological processes including pH regulation and fluid balance, highlighting their indispensable role compared to nonelectrolytes.
Summary: Key Differences Between Electrolytes and Nonelectrolytes
Electrolytes are substances that dissociate into ions when dissolved in water, enabling the solution to conduct electricity, whereas nonelectrolytes do not ionize and therefore do not conduct electricity. Key differences include electrolytes' ability to produce free ions increasing electrical conductivity, while nonelectrolytes typically dissolve as neutral molecules without ion formation. Electrolytes are often salts, acids, or bases, whereas nonelectrolytes generally consist of covalent compounds like sugar or alcohol.
Electrolyte Infographic
 libterm.com
libterm.com