An isothermal process occurs when a gas undergoes a change in volume or pressure while maintaining a constant temperature. This type of thermodynamic process follows Boyle's Law, where the product of pressure and volume remains constant. Explore the rest of the article to understand how isothermal processes impact thermodynamic systems and practical applications.
Table of Comparison
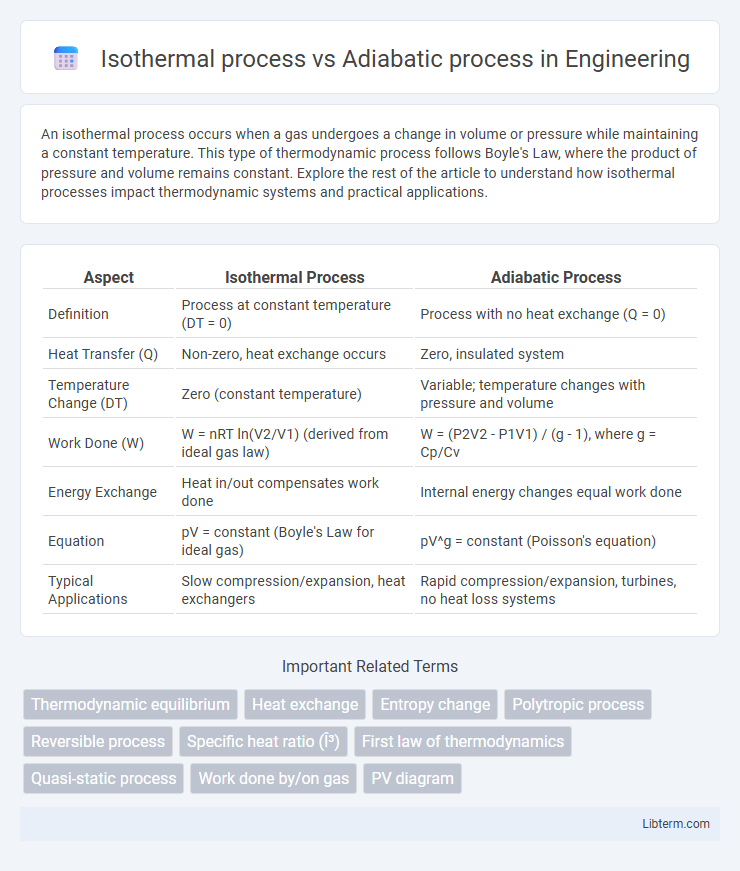
| Aspect | Isothermal Process | Adiabatic Process |
|---|---|---|
| Definition | Process at constant temperature (DT = 0) | Process with no heat exchange (Q = 0) |
| Heat Transfer (Q) | Non-zero, heat exchange occurs | Zero, insulated system |
| Temperature Change (DT) | Zero (constant temperature) | Variable; temperature changes with pressure and volume |
| Work Done (W) | W = nRT ln(V2/V1) (derived from ideal gas law) | W = (P2V2 - P1V1) / (g - 1), where g = Cp/Cv |
| Energy Exchange | Heat in/out compensates work done | Internal energy changes equal work done |
| Equation | pV = constant (Boyle's Law for ideal gas) | pV^g = constant (Poisson's equation) |
| Typical Applications | Slow compression/expansion, heat exchangers | Rapid compression/expansion, turbines, no heat loss systems |
Introduction: Understanding Thermodynamic Processes
Isothermal processes maintain constant temperature while allowing heat exchange between the system and surroundings, following the ideal gas law precisely. Adiabatic processes involve no heat transfer, with all energy changes reflected in the internal energy and temperature variations of the system. Understanding these fundamental thermodynamic processes is crucial for analyzing energy transformations in engines, refrigerators, and various engineering applications.
Definition of Isothermal Process
An isothermal process is a thermodynamic process that occurs at a constant temperature, where the internal energy of the system remains unchanged while heat exchange with the surroundings compensates for any work done. In contrast, an adiabatic process involves no heat exchange with the environment, causing the system's temperature to change as work is performed. The key distinction lies in the temperature stability of the isothermal process versus the temperature variation in the adiabatic process.
Definition of Adiabatic Process
An adiabatic process is a thermodynamic transformation in which no heat exchange occurs between the system and its surroundings, meaning the system's internal energy changes solely due to work done on or by the system. In contrast, an isothermal process occurs at a constant temperature, with heat exchange balancing work to maintain thermal equilibrium. The key characteristic of an adiabatic process is the absence of heat transfer (Q=0), often resulting in temperature and pressure changes within the system.
Key Differences: Isothermal vs Adiabatic
Isothermal processes maintain constant temperature with heat exchange between the system and surroundings, while adiabatic processes occur without heat transfer, causing temperature to change. In isothermal processes, the internal energy remains constant, resulting in pressure and volume changes governed by Boyle's Law, whereas adiabatic processes follow Poisson's equation, linking pressure, volume, and temperature variations. The key difference lies in heat exchange: isothermal allows heat flow to stabilize temperature, adiabatic isolates the system thermally, leading to internal energy changes reflected in temperature shifts.
Fundamental Equations and Laws Involved
The isothermal process follows the ideal gas law \( PV = nRT \), maintaining constant temperature where \( \Delta U = 0 \), and heat transfer \( Q \) balances the work done \( W \) by the system. The adiabatic process is governed by Poisson's equations \( PV^\gamma = \text{constant} \) and \( TV^{\gamma-1} = \text{constant} \), where \( \gamma = C_p/C_v \), and involves no heat exchange (\( Q = 0 \)), resulting in internal energy change \( \Delta U = -W \). Both processes rely on the first law of thermodynamics \( \Delta U = Q - W \), but differ fundamentally in temperature constancy and heat transfer conditions.
Practical Examples of Isothermal Processes
Isothermal processes occur when a system's temperature remains constant while heat exchange balances work done by or on the system, exemplified by the slow compression of a gas in a piston-cylinder device immersed in a thermal bath. In contrast, adiabatic processes involve no heat transfer with the surroundings, seen in rapid gas expansions in engines or atmospheric air rising quickly to form clouds. Practical examples of isothermal processes include the operation of a Stirling engine and the slow expansion of gases in refrigeration cycles, where maintaining constant temperature is critical for efficiency.
Practical Examples of Adiabatic Processes
Adiabatic processes occur when a system changes temperature without heat exchange, commonly seen in rapid gas compression in diesel engines where air heats up due to compression alone. Another practical example is in atmospheric science, where rising air expands and cools adiabatically, leading to cloud formation. Industrial applications also utilize adiabatic cooling towers, where water temperature drops as it evaporates without external heat transfer.
Graphical Representation: PV Diagrams Comparison
The graphical representation of isothermal and adiabatic processes on PV diagrams highlights distinct curves; the isothermal process features a hyperbolic curve reflecting constant temperature where PV = constant, while the adiabatic process shows a steeper curve governed by PV^g = constant, with g representing the heat capacity ratio. In an isothermal PV diagram, pressure decreases more slowly as volume increases compared to the adiabatic curve, indicating heat exchange with the surroundings. The adiabatic curve's sharper decline on the PV plot signifies no heat transfer, emphasizing internal energy changes solely due to work done by or on the system.
Applications in Real-World Engineering
Isothermal processes are essential in designing thermal systems like refrigerators and heat exchangers where temperature remains constant to ensure efficient heat transfer. Adiabatic processes are crucial in gas turbines and compressors, where no heat exchange occurs, enabling rapid pressure and temperature changes for power generation or propulsion. Both processes optimize energy efficiency and influence the thermodynamic cycle performance in various mechanical and aerospace engineering applications.
Conclusion: Choosing Between Isothermal and Adiabatic
Selecting between isothermal and adiabatic processes depends on the desired thermodynamic outcome; isothermal processes maintain constant temperature, ideal for maximizing work in slower, controlled environments. Adiabatic processes involve no heat exchange, enabling rapid changes in pressure and temperature, suitable for insulation-critical applications. Understanding their energy transfer characteristics aids in optimizing system efficiency based on specific engineering requirements.
Isothermal process Infographic
 libterm.com
libterm.com