Adhesion refers to the attractive force between different materials or surfaces, playing a crucial role in various industries such as manufacturing, construction, and healthcare. Understanding how adhesion works can improve the effectiveness of adhesives, coatings, and bonding processes for your specific applications. Explore the rest of this article to learn about key adhesion types, factors influencing adhesion strength, and practical tips to optimize bonding performance.
Table of Comparison
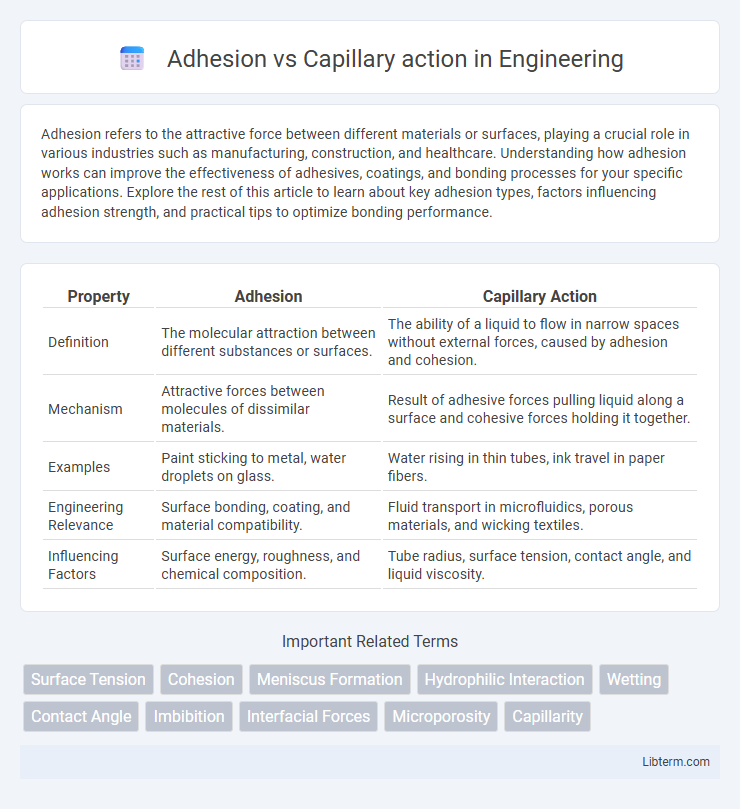
| Property | Adhesion | Capillary Action |
|---|---|---|
| Definition | The molecular attraction between different substances or surfaces. | The ability of a liquid to flow in narrow spaces without external forces, caused by adhesion and cohesion. |
| Mechanism | Attractive forces between molecules of dissimilar materials. | Result of adhesive forces pulling liquid along a surface and cohesive forces holding it together. |
| Examples | Paint sticking to metal, water droplets on glass. | Water rising in thin tubes, ink travel in paper fibers. |
| Engineering Relevance | Surface bonding, coating, and material compatibility. | Fluid transport in microfluidics, porous materials, and wicking textiles. |
| Influencing Factors | Surface energy, roughness, and chemical composition. | Tube radius, surface tension, contact angle, and liquid viscosity. |
Introduction to Adhesion and Capillary Action
Adhesion is the force of attraction between different substances, such as water molecules and the surface of a glass tube, which causes the liquid to cling to the surface. Capillary action occurs when adhesive forces between a liquid and a narrow tube or porous material, combined with cohesive forces within the liquid, enable the liquid to move upward against gravity. This phenomenon is critical in processes like water transport in plants, where adhesion to cell walls and capillary action work together to facilitate fluid movement.
Defining Adhesion: Key Concepts
Adhesion refers to the attractive force between different substances, such as water molecules and a solid surface, which enables liquids to stick to surfaces. This phenomenon is driven by intermolecular forces like hydrogen bonding and van der Waals interactions. Adhesion plays a critical role in processes such as capillary action, where liquid rises or falls within narrow spaces due to the combined effects of adhesive and cohesive forces.
Understanding Capillary Action: Core Principles
Capillary action occurs when the adhesive forces between a liquid and a solid surface exceed the cohesive forces within the liquid, causing the liquid to climb or move through narrow spaces. This phenomenon is critical in processes like water transport in plant xylem and ink movement in porous materials. Understanding capillary action hinges on the balance between adhesion to the surface and cohesion within the fluid, which drives the liquid's upward or lateral movement against gravity.
Molecular Forces Behind Adhesion
Adhesion involves molecular forces between unlike substances, primarily caused by intermolecular attractions such as hydrogen bonding, van der Waals forces, and electrostatic interactions, which enable liquids to stick to solid surfaces. Molecular forces behind adhesion differ from capillary action because adhesion specifically relates to the attraction between liquid molecules and solid surfaces, while capillary action results from a combination of adhesion and cohesion within narrow spaces. Understanding the strength and nature of these adhesive forces is essential in applications like coating, painting, and biological processes where surface interactions dictate performance.
The Science of Capillary Action in Liquids
Capillary action in liquids occurs due to the interplay between cohesive forces within the liquid and adhesive forces between the liquid and surrounding solid surfaces. Adhesion causes the liquid molecules to cling to the surface, while cohesion holds the liquid molecules together, enabling the liquid to rise or fall in narrow spaces against gravitational force. This phenomenon is critical in natural processes such as water transport in plants and fluid movement in porous materials.
Key Differences Between Adhesion and Capillary Action
Adhesion refers to the attractive force between different substances, such as water molecules and a solid surface, enabling liquids to stick to materials. Capillary action occurs when adhesion between a liquid and a narrow tube or porous material overcomes the cohesive forces within the liquid, causing it to rise or be drawn into small spaces. Key differences include that adhesion describes the force itself, whereas capillary action is a phenomenon resulting from adhesion combined with cohesion and surface tension in confined environments.
Real-World Examples of Adhesion
Adhesion is demonstrated when water droplets stick to the surface of a spider web, allowing the web to capture prey efficiently. Another example is the way paint adheres firmly to walls, ensuring durability and resistance against peeling over time. Adhesion also plays a crucial role in medical applications, such as bandages sticking securely to skin for effective wound protection.
Capillary Action in Everyday Life
Capillary action, driven by the adhesive forces between liquid molecules and surrounding solid surfaces, enables liquids to flow in narrow spaces without external forces. This phenomenon is essential in everyday life, evident in the movement of water through soil, enabling plant roots to absorb moisture and nutrients efficiently. Capillary action also plays a crucial role in ink absorption in paper and the functioning of thin-layer chromatography in laboratories.
Applications: From Biology to Engineering
Adhesion plays a critical role in biology by enabling water molecules to cling to plant cell walls, supporting nutrient transport and hydration. Capillary action is fundamental in engineering applications such as inkjet printing and microfluidic devices, where it facilitates precise fluid movement through narrow channels without external pumps. Both phenomena are essential in soil moisture retention and construction materials, influencing water absorption and structural integrity.
Summary: Comparing Adhesion vs Capillary Action
Adhesion refers to the attractive forces between different substances, such as water molecules binding to a glass surface, while capillary action is the movement of liquid within narrow spaces caused by adhesive and cohesive forces. Capillary action depends on both adhesion between the liquid and the solid surface and the surface tension of the liquid, enabling it to rise or fall in tubes or porous materials. The key difference lies in adhesion being a fundamental force, whereas capillary action is a phenomenon resulting from the interplay of adhesion, cohesion, and surface tension.
Adhesion Infographic
 libterm.com
libterm.com