The Otto cycle is the fundamental thermodynamic process that describes the operation of a spark-ignition internal combustion engine, where air-fuel mixture undergoes compression, combustion, expansion, and exhaust. Efficiency improvements in the Otto cycle directly impact your vehicle's performance and fuel economy by optimizing these stages. Explore the rest of the article to understand how this cycle influences modern engine technology and efficiency.
Table of Comparison
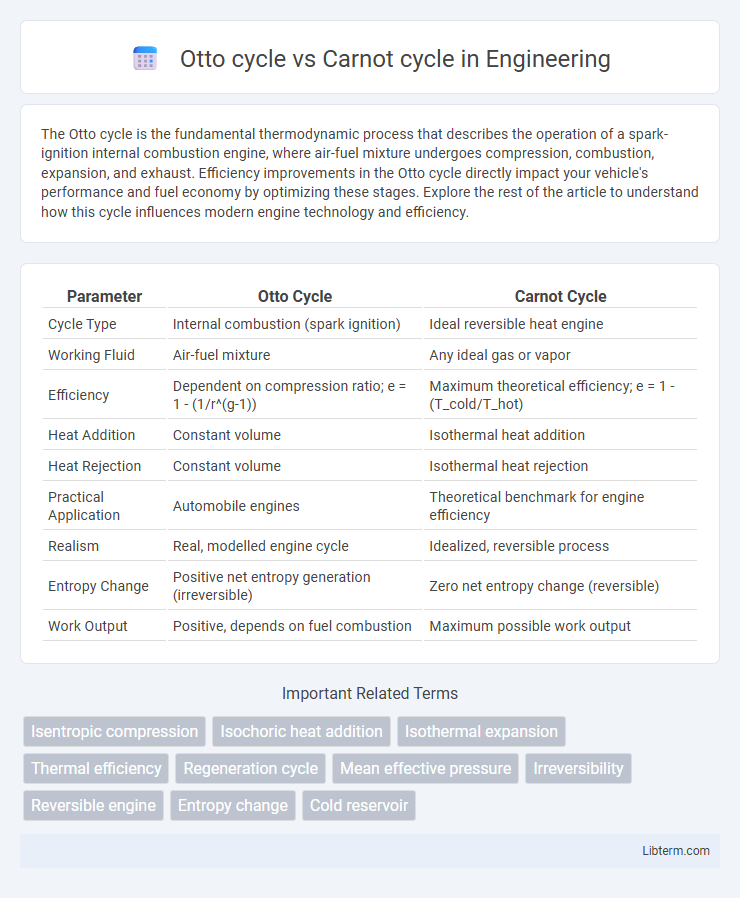
| Parameter | Otto Cycle | Carnot Cycle |
|---|---|---|
| Cycle Type | Internal combustion (spark ignition) | Ideal reversible heat engine |
| Working Fluid | Air-fuel mixture | Any ideal gas or vapor |
| Efficiency | Dependent on compression ratio; e = 1 - (1/r^(g-1)) | Maximum theoretical efficiency; e = 1 - (T_cold/T_hot) |
| Heat Addition | Constant volume | Isothermal heat addition |
| Heat Rejection | Constant volume | Isothermal heat rejection |
| Practical Application | Automobile engines | Theoretical benchmark for engine efficiency |
| Realism | Real, modelled engine cycle | Idealized, reversible process |
| Entropy Change | Positive net entropy generation (irreversible) | Zero net entropy change (reversible) |
| Work Output | Positive, depends on fuel combustion | Maximum possible work output |
Introduction to Otto Cycle and Carnot Cycle
The Otto cycle, fundamental to spark-ignition internal combustion engines, operates through isentropic compression and expansion processes combined with constant-volume heat addition and rejection. The Carnot cycle represents an idealized thermodynamic cycle with maximum efficiency, consisting of two isothermal and two adiabatic processes, serving as a benchmark for all heat engine cycles. Comparing these cycles highlights the practical applications and theoretical limits in thermal efficiency and engine performance analysis.
Fundamental Concepts of Thermodynamic Cycles
The Otto cycle represents the idealized thermodynamic process for spark-ignition internal combustion engines, characterized by two adiabatic and two isochoric processes, emphasizing rapid combustion at constant volume. In contrast, the Carnot cycle embodies the theoretical maximum efficiency boundary for all heat engines operating between two thermal reservoirs, involving two isothermal and two adiabatic processes. Fundamental distinctions lie in their reversible heat transfer mechanisms and practical applicability, with the Carnot cycle defining efficiency limits while the Otto cycle models real-world engine behavior.
Working Principles of the Otto Cycle
The Otto cycle operates on a four-stroke internal combustion process featuring two adiabatic and two isochoric processes, converting chemical energy into mechanical work through controlled combustion of the air-fuel mixture. Unlike the Carnot cycle, which is idealized with isothermal and adiabatic processes, the Otto cycle's efficiency depends largely on the compression ratio and the specific heat ratio of the working gas. Key parameters such as pressure, volume, and temperature changes during the compression and expansion strokes define the thermodynamic behavior and overall performance of the Otto cycle engine.
Carnot Cycle: Process and Key Features
The Carnot cycle consists of four reversible processes: two isothermal processes where heat transfer occurs at constant temperature, and two adiabatic processes with no heat exchange. This cycle operates between two thermal reservoirs, achieving maximum theoretical efficiency based solely on the temperature difference, making it an ideal benchmark for heat engines. Key features include its reversible nature, reliance on entropy changes during isothermal expansions and compressions, and its role in establishing the upper limit of efficiency for any real thermodynamic engine.
Efficiency Comparison: Otto Cycle vs Carnot Cycle
The Carnot cycle represents the maximum theoretical efficiency achievable between two temperatures, defined by 1 - (T_cold/T_hot), making it the benchmark for all heat engines. The Otto cycle, typical in gasoline engines, has lower efficiency due to its limitation by the compression ratio and specific heat capacities of the working gas, resulting in practical efficiencies significantly below the Carnot limit. Comparing them highlights that while the Carnot cycle sets the upper efficiency bound, real Otto cycle engines operate with reduced efficiency because of irreversibilities and non-ideal processes.
Assumptions and Real-world Applications
The Otto cycle assumes a constant volume heat addition and is idealized for spark-ignition internal combustion engines, whereas the Carnot cycle assumes reversible isothermal and adiabatic processes, representing the maximum possible efficiency between two temperature reservoirs. Otto cycle models account for practical engine processes like combustion and expansion phases, making them more applicable to real-world gasoline engines despite lower ideal efficiency compared to the Carnot cycle. The Carnot cycle serves as a theoretical benchmark for heat engine efficiency but is not directly implementable due to its idealized assumptions of reversible and frictionless processes.
Thermodynamic Diagrams: P-V and T-S Representations
The Otto cycle, depicted on P-V diagrams by distinct isochoric and adiabatic processes, shows sharp pressure rises and drops corresponding to combustion and exhaust strokes, while the T-S diagram illustrates entropy changes primarily during heat addition and rejection at constant volume. In contrast, the Carnot cycle's P-V diagram features two isothermal and two adiabatic processes representing idealized reversible heat engine stages, and its T-S diagram forms a perfect rectangle, indicating isentropic expansion and compression coupled with isothermal heat transfer. These thermodynamic diagrams highlight the Otto cycle's practical engine operation with irreversibilities versus the Carnot cycle's theoretical efficiency limits and reversible processes.
Advantages and Disadvantages of Each Cycle
The Otto cycle offers higher power output and simpler engine design, making it more practical for internal combustion engines, but it suffers from lower thermal efficiency compared to the Carnot cycle. The Carnot cycle achieves the maximum possible thermal efficiency between two temperature limits, serving as an ideal benchmark, yet it is impractical for real engines due to its reversible processes and requirement for infinitely slow operation. While the Otto cycle balances efficiency and feasibility in real-world applications, the Carnot cycle remains a theoretical model highlighting the upper limit of heat engine performance.
Practical Limitations and Challenges
The Otto cycle, commonly used in internal combustion engines, faces practical limitations such as incomplete combustion and heat losses, which reduce its efficiency compared to the idealized Carnot cycle. The Carnot cycle represents the maximum theoretical efficiency between two temperature reservoirs but is impractical due to reversible processes requiring infinitely slow operation and ideal insulation. Mechanical complexities, friction, and real fuel properties further challenge achieving Carnot efficiency in practical engines, making the Otto cycle a more feasible but less efficient alternative.
Conclusion: Which Cycle is Superior?
The Carnot cycle is thermodynamically superior due to its theoretical maximum efficiency based on reversible processes and an idealized heat engine operating between two temperature reservoirs. The Otto cycle, commonly used in internal combustion engines, offers practical application advantages but operates with lower efficiency due to irreversibilities and real gas behavior. Therefore, the Carnot cycle represents the efficiency benchmark, while the Otto cycle provides a realistic model for engine performance within practical constraints.
Otto cycle Infographic
 libterm.com
libterm.com