Anodizing is an electrochemical process that enhances the natural oxide layer on metal surfaces, primarily aluminum, improving corrosion resistance, wear durability, and aesthetic appeal. This technique creates a porous, durable coating that can be dyed in various colors, making it popular in both industrial and decorative applications. Discover how anodizing can protect and beautify your metal products by reading the rest of the article.
Table of Comparison
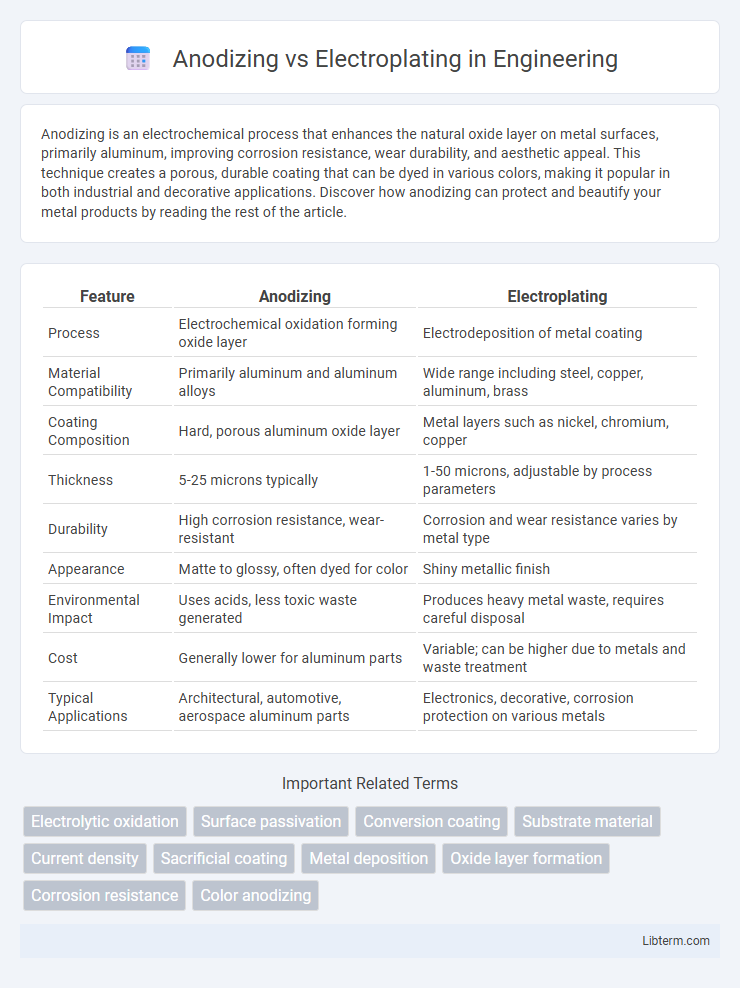
| Feature | Anodizing | Electroplating |
|---|---|---|
| Process | Electrochemical oxidation forming oxide layer | Electrodeposition of metal coating |
| Material Compatibility | Primarily aluminum and aluminum alloys | Wide range including steel, copper, aluminum, brass |
| Coating Composition | Hard, porous aluminum oxide layer | Metal layers such as nickel, chromium, copper |
| Thickness | 5-25 microns typically | 1-50 microns, adjustable by process parameters |
| Durability | High corrosion resistance, wear-resistant | Corrosion and wear resistance varies by metal type |
| Appearance | Matte to glossy, often dyed for color | Shiny metallic finish |
| Environmental Impact | Uses acids, less toxic waste generated | Produces heavy metal waste, requires careful disposal |
| Cost | Generally lower for aluminum parts | Variable; can be higher due to metals and waste treatment |
| Typical Applications | Architectural, automotive, aerospace aluminum parts | Electronics, decorative, corrosion protection on various metals |
Introduction to Surface Finishing Techniques
Anodizing and electroplating are essential surface finishing techniques that enhance metal properties by creating protective and decorative coatings. Anodizing primarily modifies aluminum through an electrochemical process that thickens the natural oxide layer, increasing corrosion resistance and durability. Electroplating deposits a thin layer of metal, such as nickel, chrome, or gold, onto a substrate to improve wear resistance, appearance, and electrical conductivity.
What is Anodizing?
Anodizing is an electrochemical process that enhances the natural oxide layer on aluminum surfaces, improving corrosion resistance and surface durability. This technique creates a porous, protective coating that can be dyed for aesthetic purposes while maintaining excellent adhesion. Unlike electroplating, which deposits a metal layer on the substrate, anodizing modifies the metal itself, resulting in a more robust and wear-resistant finish.
What is Electroplating?
Electroplating is a metal finishing process that uses an electric current to deposit a thin layer of metal onto the surface of a conductive object, enhancing corrosion resistance, surface hardness, and aesthetic appeal. Common metals used in electroplating include nickel, chromium, gold, and silver, each providing specific functional or decorative benefits. Unlike anodizing, which forms an oxide layer on the metal itself, electroplating adds a distinct metallic coating, improving both durability and conductivity.
Key Differences Between Anodizing and Electroplating
Anodizing is an electrochemical process that thickens the natural oxide layer on aluminum surfaces, enhancing corrosion resistance and wear, while electroplating deposits a thin metal coating, such as chromium or nickel, onto a substrate to improve appearance and conductivity. Anodizing only applies to non-ferrous metals like aluminum and titanium, creating a porous, corrosion-resistant surface, whereas electroplating can coat a variety of metals and plastics with a protective or decorative metallic layer. The durability of anodized layers lies in their integration with the base metal, whereas electroplated surfaces rely on the adhesion of the deposited metal, which may be prone to chipping or peeling.
Materials Used in Anodizing vs Electroplating
Anodizing primarily involves aluminum and its alloys, utilizing an electrolytic process to form a durable oxide layer that enhances corrosion resistance and surface hardness. Electroplating applies various metals such as nickel, chromium, copper, and gold onto substrates like steel, brass, and plastic for improved appearance, conductivity, and protection. Material selection depends on the desired properties, with anodizing limited to reactive metals and electroplating offering broader compatibility across diverse base materials and coating metals.
Advantages of Anodizing
Anodizing enhances corrosion resistance by creating a durable oxide layer on aluminum surfaces, which is thicker and more resistant than electroplated coatings. It provides superior wear resistance and improved adhesion for paints and adhesives, making it ideal for aerospace and architectural applications. Anodized surfaces are also environmentally friendly, using fewer harmful chemicals compared to electroplating processes that often involve toxic metals.
Benefits of Electroplating
Electroplating enhances corrosion resistance and improves surface hardness, extending the lifespan of metal components in automotive, aerospace, and electronics industries. It provides uniform metal coatings, allowing for precise control over thickness and appearance, which is essential for decorative and functional applications. Additionally, electroplating enables the use of less expensive base metals while maintaining the desired aesthetic and protective properties.
Common Applications of Anodizing and Electroplating
Anodizing is widely used in industries requiring enhanced corrosion resistance and surface hardness, such as aerospace, automotive, and consumer electronics, where aluminum components benefit from protective oxide layers. Electroplating finds common applications in jewelry, decorative items, and electrical contacts, providing metal coatings like gold, silver, or nickel to improve appearance, conductivity, and wear resistance. Both processes serve critical roles in manufacturing, with anodizing favored for aluminum alloys and electroplating applied to a variety of metals including steel and copper.
Cost Comparison: Anodizing vs Electroplating
Anodizing typically offers a lower overall cost than electroplating due to its simpler process and lower material expenses, especially for aluminum parts. Electroplating costs can escalate with the use of precious metals like gold or silver and require more complex waste disposal measures. For large-scale production, anodizing provides a cost-effective, durable surface finish, while electroplating may be justified for specific decorative or corrosion resistance needs despite higher costs.
Choosing the Right Process for Your Needs
Selecting between anodizing and electroplating depends on the desired surface properties and application requirements. Anodizing enhances corrosion resistance and wear by forming a durable oxide layer, ideal for aluminum parts needing anodic protection. Electroplating deposits a metal layer, such as chrome or nickel, providing superior conductivity, decorative finishes, and increased hardness, suitable for components requiring enhanced electrical properties or aesthetic appeal.
Anodizing Infographic
 libterm.com
libterm.com