Galvanizing involves applying a protective zinc coating to steel or iron to prevent rust and corrosion, extending the durability of your metal structures. This process is essential in construction, automotive, and industrial applications where long-lasting strength is required. Explore the rest of this article to learn how galvanizing can enhance your projects and the different methods available.
Table of Comparison
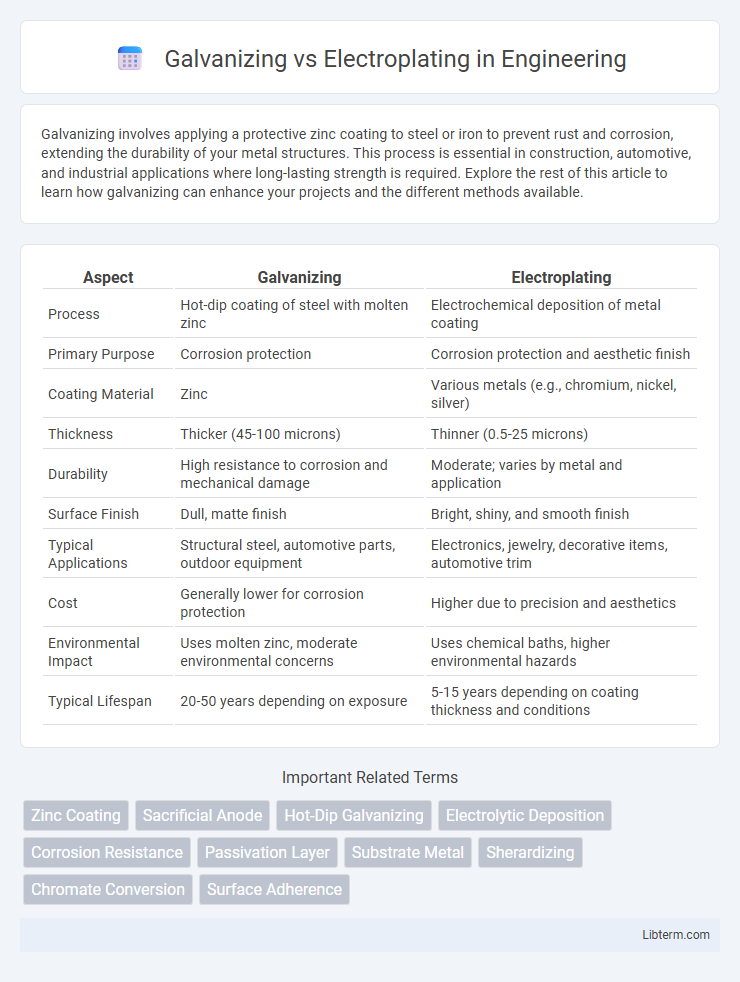
| Aspect | Galvanizing | Electroplating |
|---|---|---|
| Process | Hot-dip coating of steel with molten zinc | Electrochemical deposition of metal coating |
| Primary Purpose | Corrosion protection | Corrosion protection and aesthetic finish |
| Coating Material | Zinc | Various metals (e.g., chromium, nickel, silver) |
| Thickness | Thicker (45-100 microns) | Thinner (0.5-25 microns) |
| Durability | High resistance to corrosion and mechanical damage | Moderate; varies by metal and application |
| Surface Finish | Dull, matte finish | Bright, shiny, and smooth finish |
| Typical Applications | Structural steel, automotive parts, outdoor equipment | Electronics, jewelry, decorative items, automotive trim |
| Cost | Generally lower for corrosion protection | Higher due to precision and aesthetics |
| Environmental Impact | Uses molten zinc, moderate environmental concerns | Uses chemical baths, higher environmental hazards |
| Typical Lifespan | 20-50 years depending on exposure | 5-15 years depending on coating thickness and conditions |
Introduction to Galvanizing and Electroplating
Galvanizing involves coating steel or iron with a protective layer of zinc through a hot-dip process, enhancing corrosion resistance and durability. Electroplating uses an electrical current to deposit a thin metal layer, such as chromium or nickel, onto a conductive surface to improve appearance and prevent rust. Both methods serve protective and decorative purposes but differ in application techniques and thickness of the coating.
Core Principles: How Galvanizing Works
Galvanizing involves coating steel or iron with a protective layer of zinc through a hot-dip process, where the metal is immersed in molten zinc, forming a metallurgical bond that prevents corrosion. The zinc coating acts as a sacrificial anode, corroding before the base metal and extending its lifespan. This method creates a durable, abrasion-resistant finish ideal for outdoor and industrial applications.
Electroplating Explained: The Science Behind the Process
Electroplating involves using an electric current to reduce dissolved metal cations, allowing them to form a coherent metal coating on a conductive surface, which enhances corrosion resistance, improves aesthetic appeal, and increases surface hardness. The process relies on an electrolyte solution containing metal salts, where the object to be plated acts as the cathode and a metal anode dissolves to replenish ions. Precise control of voltage, current density, and electrolyte composition ensures uniform deposition and strong adhesion of the metal layer.
Materials and Metals: Suitable Surfaces for Each Method
Galvanizing primarily suits ferrous metals such as steel and iron, creating a durable zinc coating that prevents rust and corrosion. Electroplating applies to a broader range of materials, including metals like copper, zinc, nickel, and precious metals, enhancing surface properties such as conductivity and appearance. The choice between the two depends on the substrate material and desired protective or decorative finish.
Protective Capabilities: Corrosion Resistance Comparison
Galvanizing provides superior corrosion resistance by forming a robust zinc coating that sacrificially protects steel from rust, making it ideal for outdoor and industrial applications. Electroplating offers a thinner metal layer, which can be precisely controlled for decorative or conductive purposes but generally provides less long-term corrosion protection than galvanizing. The zinc oxide layer formed during galvanizing acts as a durable barrier against moisture and environmental elements, whereas electroplated coatings may require additional maintenance to sustain protective qualities.
Surface Finish and Aesthetics: Appearance Differences
Galvanizing produces a matte, rough-textured surface with a thicker zinc coating that enhances corrosion resistance but offers limited aesthetic appeal compared to electroplating. Electroplating provides a smooth, glossy, and visually appealing finish with precise control over layer thickness and color, making it ideal for decorative purposes. The choice between galvanizing and electroplating significantly impacts the visual quality and durability of metal surfaces in various applications.
Durability and Longevity: Which Lasts Longer?
Galvanizing typically provides superior durability and longevity compared to electroplating due to its thick zinc coating that forms a robust barrier against corrosion and physical wear. Electroplating offers a thinner metal layer, which can wear off faster under harsh environmental conditions, reducing its lifespan. In industrial and outdoor applications, galvanized coatings often last 20 to 50 years, significantly outlasting electroplated finishes that may only endure a few years before requiring maintenance.
Cost Analysis: Galvanizing vs Electroplating Expenses
Galvanizing generally involves higher initial setup costs due to the extensive equipment and zinc material required, but offers lower long-term maintenance expenses because of its durable, corrosion-resistant coating. Electroplating typically has lower upfront costs and allows for precise, thin metal layers, yet it often requires more frequent reapplication and generates hazardous waste disposal expenses. Choosing between galvanizing and electroplating hinges on balancing initial investment against ongoing maintenance and environmental compliance costs.
Environmental Impact and Safety Considerations
Galvanizing involves coating steel with a layer of zinc through hot-dip immersion, producing a durable, corrosion-resistant finish with minimal hazardous waste, while electroplating uses an electric current to deposit metals like nickel or chromium, often generating toxic chemical effluents requiring strict handling. The galvanizing process emits fewer volatile organic compounds and heavy metals compared to electroplating, reducing environmental contamination risks and improving workplace air quality. Safety considerations favor galvanizing due to lower exposure to harmful chemicals and simpler waste management, whereas electroplating demands stringent controls to prevent chemical spills and worker exposure to hazardous substances.
Choosing the Right Coating Method for Your Application
Galvanizing provides a thick, durable zinc coating ideal for protecting steel from corrosion in outdoor or industrial environments, while electroplating offers a thinner, decorative metal layer suited for aesthetic enhancement and precise thickness control. Selecting the right coating method depends on factors such as environmental exposure, required corrosion resistance, and desired finish quality. For heavy-duty protection where longevity is critical, galvanizing is preferable; for applications demanding fine detailing and varied metal finishes, electroplating is more suitable.
Galvanizing Infographic
 libterm.com
libterm.com