Boiling point elevation occurs when a non-volatile solute is dissolved in a solvent, causing the solution's boiling temperature to rise above that of the pure solvent due to decreased vapor pressure. This colligative property is essential in understanding how solute concentration affects phase changes and is widely applied in chemistry and chemical engineering. Explore the article further to learn how this phenomenon impacts everyday processes and industrial applications.
Table of Comparison
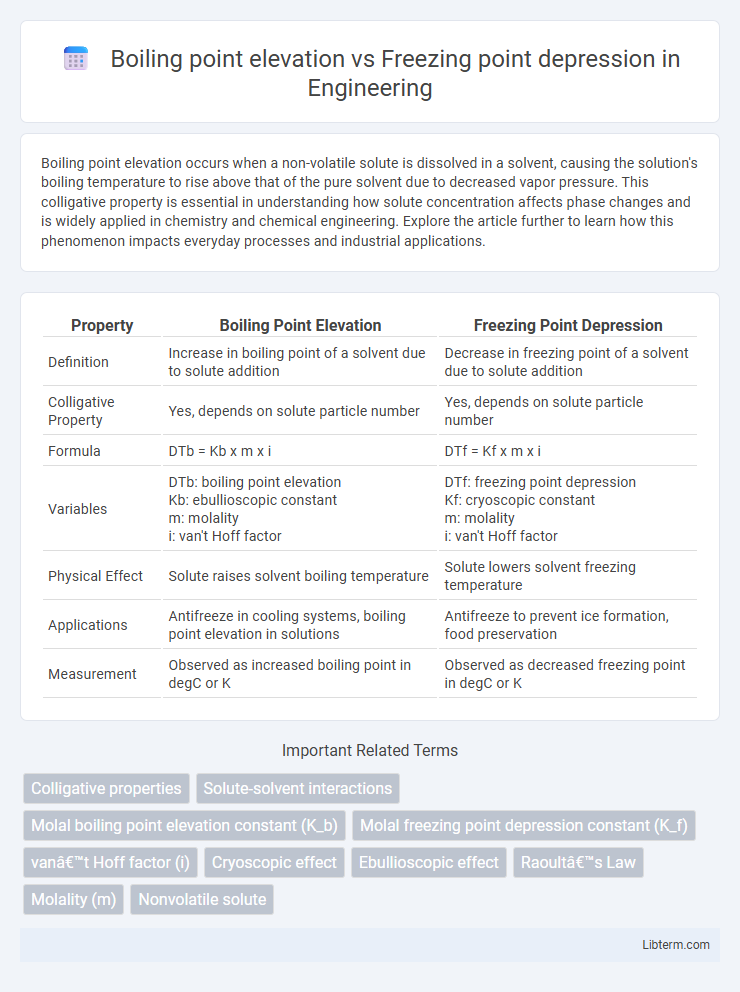
| Property | Boiling Point Elevation | Freezing Point Depression |
|---|---|---|
| Definition | Increase in boiling point of a solvent due to solute addition | Decrease in freezing point of a solvent due to solute addition |
| Colligative Property | Yes, depends on solute particle number | Yes, depends on solute particle number |
| Formula | DTb = Kb x m x i | DTf = Kf x m x i |
| Variables | DTb: boiling point elevation Kb: ebullioscopic constant m: molality i: van't Hoff factor |
DTf: freezing point depression Kf: cryoscopic constant m: molality i: van't Hoff factor |
| Physical Effect | Solute raises solvent boiling temperature | Solute lowers solvent freezing temperature |
| Applications | Antifreeze in cooling systems, boiling point elevation in solutions | Antifreeze to prevent ice formation, food preservation |
| Measurement | Observed as increased boiling point in degC or K | Observed as decreased freezing point in degC or K |
Introduction to Colligative Properties
Boiling point elevation and freezing point depression are key colligative properties that depend on the number of solute particles dissolved in a solvent, rather than their chemical identity. These properties arise due to the disruption of solvent molecules' phase equilibrium caused by solute presence, leading to higher boiling points and lower freezing points in solutions. Understanding the quantitative relationship of colligative effects through molality and van't Hoff factor enables precise calculation of solution behavior in various chemical and industrial applications.
Defining Boiling Point Elevation
Boiling point elevation occurs when a solute is dissolved in a solvent, causing the solution's boiling temperature to increase compared to the pure solvent. This phenomenon results from the lowering of the solvent's vapor pressure, requiring higher temperatures to reach the boiling point. Boiling point elevation is quantitatively described by the equation DTb = iKb*m, where DTb is the boiling point elevation, i is the van't Hoff factor, Kb is the solvent's ebullioscopic constant, and m is the molality of the solution.
Understanding Freezing Point Depression
Freezing point depression occurs when a solute is added to a solvent, lowering the temperature at which the liquid solidifies due to decreased solvent vapor pressure. This colligative property depends on the number of solute particles rather than their identity, making it important in processes such as antifreeze formulation and salt application on icy roads. Understanding freezing point depression involves calculating the temperature change using the molal freezing point depression constant (K_f) and solute concentration in molality.
Molecular Basis of Boiling Point Elevation
Boiling point elevation occurs because the addition of solute particles disrupts the vapor pressure of a solvent, requiring higher temperatures to reach boiling. On a molecular level, solute molecules interfere with the kinetic energy of solvent molecules, reducing their ability to escape into the gas phase. This colligative property depends on the number of solute particles and is directly proportional to the molal concentration of the solution.
Mechanism Behind Freezing Point Depression
Freezing point depression occurs when solute particles disrupt the formation of a solid lattice in a solvent, lowering the temperature required for the solvent to solidify. This colligative property arises because dissolved solutes decrease the solvent's chemical potential, preventing molecules from organizing into a solid phase. Unlike boiling point elevation, which involves increased vapor pressure, freezing point depression specifically results from interference in the crystallization process.
Key Differences Between Boiling Point Elevation and Freezing Point Depression
Boiling point elevation occurs when a solute is added to a solvent, causing the boiling temperature to increase due to decreased vapor pressure, while freezing point depression results in a lowered freezing temperature by disrupting the solvent's crystallization process. The magnitude of boiling point elevation is quantified by the ebullioscopic constant (Kb), whereas freezing point depression is measured using the cryoscopic constant (Kf), both specific to the solvent. Unlike freezing point depression, boiling point elevation requires heating to overcome the elevated boiling point, highlighting a fundamental difference in the phase changes involved and their thermodynamic implications.
Factors Affecting Both Phenomena
Boiling point elevation and freezing point depression both depend on solute concentration, specifically the molality of the solution, with higher molality causing greater shifts in phase change temperatures. The nature of the solute also impacts these colligative properties; non-volatile, non-electrolyte solutes produce more predictable changes, while electrolytes cause larger effects due to ion dissociation and increased particle number. Temperature, solvent identity, and atmospheric pressure influence the magnitude of boiling point elevation and freezing point depression, as variations in vapor pressure and solvent-solute interactions affect the thermal dynamics of the solution.
Real-World Applications and Examples
Boiling point elevation is crucial in antifreeze formulations for vehicle engines, preventing overheating by raising the coolant's boiling temperature, while freezing point depression is essential in de-icing roads, lowering the freezing temperature of water to avoid ice formation. In pharmaceutical manufacturing, boiling point elevation helps control solvent evaporation rates during drug formulation, whereas freezing point depression aids cryopreservation by preventing ice crystal formation in biological samples. These principles also apply in food industry processes, where boiling point elevation improves candy-making by increasing syrup concentration without premature boiling, and freezing point depression maintains texture in frozen desserts by inhibiting ice crystallization.
Mathematical Expressions and Calculations
Boiling point elevation and freezing point depression are colligative properties quantified by the formulas DTb = iKb m and DTf = iKf m respectively, where DTb and DTf represent the changes in boiling and freezing points, i is the van't Hoff factor, Kb and Kf are the solvent's boiling point elevation and freezing point depression constants, and m denotes the molality of the solution. Calculations often involve determining the molality from solute mass and solvent mass, then applying these values to find the exact temperature shift caused by solute addition. Precise use of these equations enables quantification of solution behavior under temperature changes essential for fields like chemistry and chemical engineering.
Summary and Practical Implications
Boiling point elevation and freezing point depression are colligative properties that depend on solute particle concentration rather than chemical identity, crucial for determining solution behavior in chemistry and engineering. Boiling point elevation increases the boiling temperature of a solvent, used in applications like antifreeze formulation and cooking processes, while freezing point depression lowers the freezing temperature, essential for de-icing roads and preserving biological samples. Understanding these phenomena enables precise control over phase transitions in industrial manufacturing, pharmaceuticals, and environmental science.
Boiling point elevation Infographic
 libterm.com
libterm.com