Colligative properties depend on the number of solute particles in a solvent rather than their identity, influencing phenomena such as boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. These properties play vital roles in everyday applications like cooking, antifreeze formulation, and medical treatments involving osmosis. Explore the rest of the article to deepen your understanding of how colligative properties impact various scientific and practical fields.
Table of Comparison
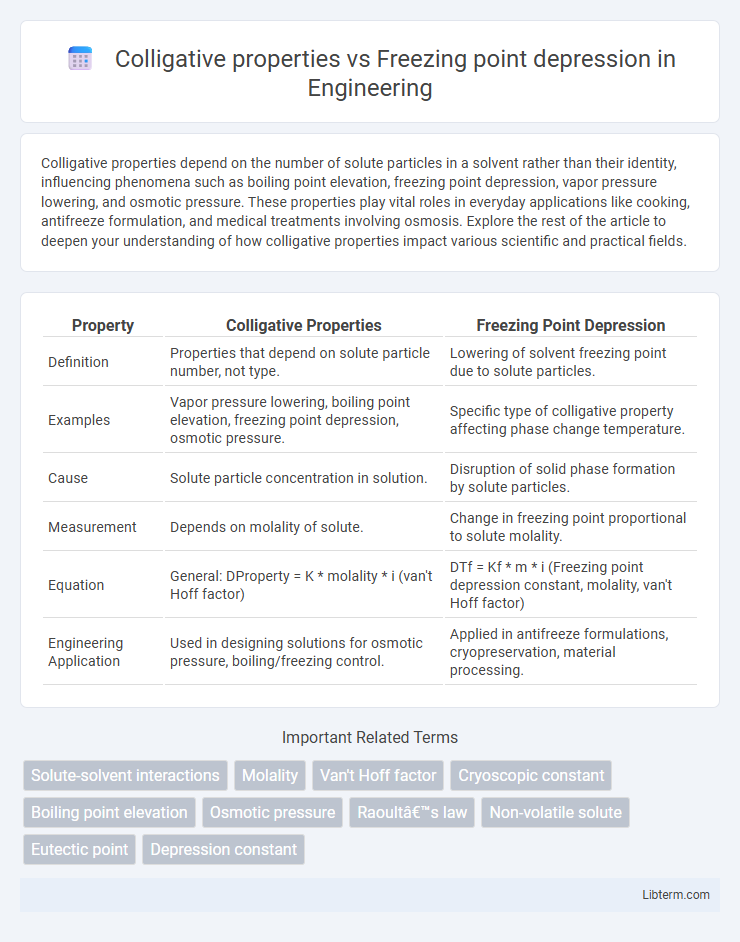
| Property | Colligative Properties | Freezing Point Depression |
|---|---|---|
| Definition | Properties that depend on solute particle number, not type. | Lowering of solvent freezing point due to solute particles. |
| Examples | Vapor pressure lowering, boiling point elevation, freezing point depression, osmotic pressure. | Specific type of colligative property affecting phase change temperature. |
| Cause | Solute particle concentration in solution. | Disruption of solid phase formation by solute particles. |
| Measurement | Depends on molality of solute. | Change in freezing point proportional to solute molality. |
| Equation | General: DProperty = K * molality * i (van't Hoff factor) | DTf = Kf * m * i (Freezing point depression constant, molality, van't Hoff factor) |
| Engineering Application | Used in designing solutions for osmotic pressure, boiling/freezing control. | Applied in antifreeze formulations, cryopreservation, material processing. |
Introduction to Colligative Properties
Colligative properties depend on the number of solute particles in a solution rather than their identity, encompassing boiling point elevation, vapor pressure lowering, osmotic pressure, and freezing point depression. Freezing point depression occurs when solute particles disrupt the formation of a solid lattice, causing a solution's freezing point to be lower than that of the pure solvent. Understanding colligative properties is essential for applications in chemistry and biology, such as determining molar mass and controlling cryopreservation processes.
Understanding Freezing Point Depression
Freezing point depression is a colligative property that occurs when a solute is dissolved in a solvent, lowering the temperature at which the solution freezes compared to the pure solvent. This phenomenon is directly proportional to the molal concentration of the solute particles and depends on the solvent's freezing point depression constant (Kf). Understanding freezing point depression is crucial in fields like chemistry and environmental science for predicting solution behavior and preventing ice formation in antifreeze applications.
Key Differences: Colligative Properties vs Freezing Point Depression
Colligative properties refer to the set of physical changes in a solvent caused by the presence of solute particles, including vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. Freezing point depression specifically measures the decrease in the freezing temperature of a solvent when a solute is dissolved, making it a subset of colligative properties. The key difference lies in colligative properties encompassing multiple effects based purely on solute particle concentration, whereas freezing point depression focuses exclusively on the freezing temperature shift.
The Science Behind Colligative Properties
Colligative properties depend on the number of solute particles in a solvent rather than their identity, influencing physical changes such as vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. Freezing point depression occurs because solute particles disrupt the formation of the solid lattice structure in the solvent, requiring a lower temperature to solidify. These properties are essential for calculating molar masses and understanding solution behaviors in chemistry and materials science.
Factors Influencing Freezing Point Depression
Freezing point depression occurs when solute particles disrupt the formation of a solid lattice, lowering the temperature at which a solvent freezes. Factors influencing freezing point depression include the molal concentration of the solute, the nature of the solute particles (ionic or molecular), and the solvent's inherent freezing point. Colligative properties depend solely on the number of solute particles, making electrolyte solutions, which dissociate into multiple ions, cause greater freezing point depression than nonelectrolyte solutions.
Real-World Applications of Colligative Properties
Colligative properties, including freezing point depression, play a crucial role in real-world applications such as antifreeze formulations for automotive engines and de-icing roads during winter. Freezing point depression occurs when solute particles disrupt the equilibrium of water molecules, lowering the temperature at which the liquid freezes, thereby preventing ice formation. This principle is also essential in cryopreservation techniques used in biological and medical fields to preserve cells and tissues at subzero temperatures without ice damage.
Calculating Freezing Point Depression
Freezing point depression is a colligative property that quantifies the decrease in a solvent's freezing point due to the presence of solute particles. It is calculated using the formula DTf = i x Kf x m, where DTf represents the freezing point depression, i is the van't Hoff factor indicating the number of particles the solute dissociates into, Kf is the cryoscopic constant specific to the solvent, and m denotes the molality of the solution. The calculation highlights the direct dependence on solute concentration and the nature of both solute and solvent, making freezing point depression a key example of colligative properties in solution chemistry.
Colligative Properties in Everyday Life
Colligative properties, including freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure, are essential in everyday life for processes like antifreeze in vehicles and salt on icy roads. Freezing point depression specifically explains how adding solutes like salt lowers the freezing point of water, preventing ice formation. Understanding these properties helps in applications ranging from food preservation to medical treatments such as intravenous fluids.
Freezing Point Depression: Practical Examples
Freezing point depression is a colligative property where the addition of a solute lowers the freezing point of a solvent, demonstrated by practical examples like salt on icy roads preventing water from freezing. In antifreeze solutions, ethylene glycol lowers the freezing point of water in car radiators, enhancing cold-weather performance. Ocean water's freezing point is reduced due to salt content, allowing it to remain liquid at temperatures below 0degC.
Summary: Relating Colligative Properties to Freezing Point Depression
Colligative properties depend on the number of solute particles in a solvent rather than their identity, directly influencing freezing point depression by lowering the temperature at which a solution freezes. Freezing point depression occurs because solute particles disrupt the formation of the solid phase, requiring a colder temperature to overcome this interference. The relationship between colligative properties and freezing point depression is quantitatively described by DTf = iKfm, where DTf is the freezing point depression, i is the van't Hoff factor, Kf the cryoscopic constant, and m the molality of the solution.
Colligative properties Infographic
 libterm.com
libterm.com