Osmotic pressure is the force exerted by a solvent as it moves through a semipermeable membrane toward a higher concentration solution. It plays a crucial role in biological systems, influencing cell hydration, nutrient absorption, and waste removal. Explore this article to understand how osmotic pressure affects your body and everyday life.
Table of Comparison
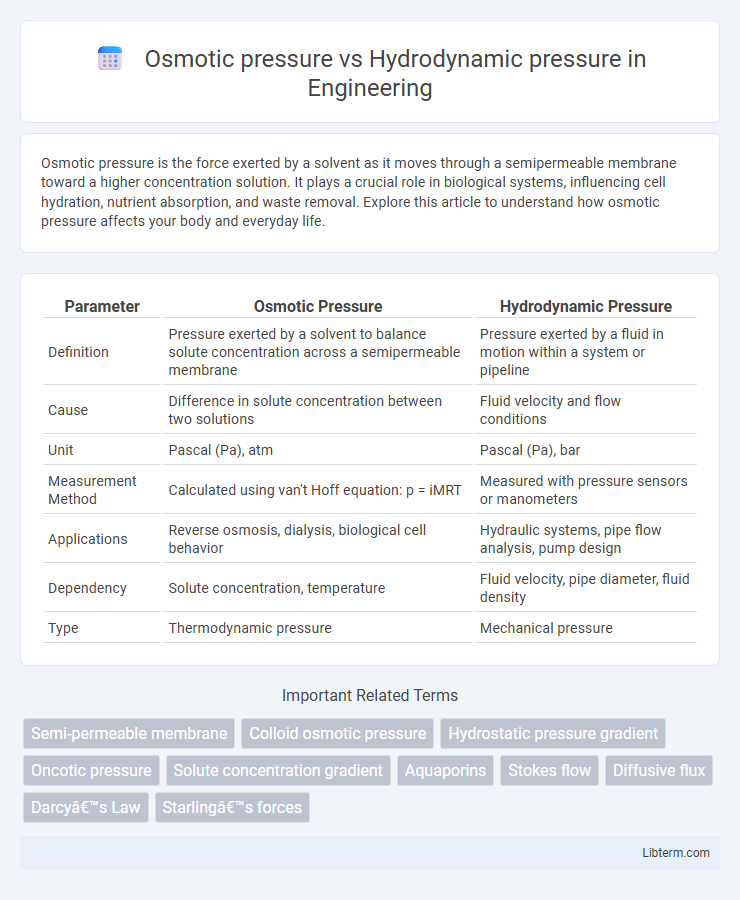
| Parameter | Osmotic Pressure | Hydrodynamic Pressure |
|---|---|---|
| Definition | Pressure exerted by a solvent to balance solute concentration across a semipermeable membrane | Pressure exerted by a fluid in motion within a system or pipeline |
| Cause | Difference in solute concentration between two solutions | Fluid velocity and flow conditions |
| Unit | Pascal (Pa), atm | Pascal (Pa), bar |
| Measurement Method | Calculated using van't Hoff equation: p = iMRT | Measured with pressure sensors or manometers |
| Applications | Reverse osmosis, dialysis, biological cell behavior | Hydraulic systems, pipe flow analysis, pump design |
| Dependency | Solute concentration, temperature | Fluid velocity, pipe diameter, fluid density |
| Type | Thermodynamic pressure | Mechanical pressure |
Introduction to Osmotic and Hydrodynamic Pressure
Osmotic pressure arises from the difference in solute concentration across a semipermeable membrane, driving solvent molecules to move and equalize concentrations. Hydrodynamic pressure, on the other hand, results from the physical force exerted by a fluid in motion within a confined space or system. Understanding the distinction between osmotic and hydrodynamic pressures is essential in fields such as fluid dynamics, biology, and chemical engineering, where membrane transport and fluid flow are critical processes.
Defining Osmotic Pressure: Key Concepts
Osmotic pressure is the force exerted by the movement of solvent molecules through a semipermeable membrane from a region of lower solute concentration to higher solute concentration, aiming to equalize solute levels on both sides. It depends on solute concentration, temperature, and the nature of the solvent, typically described by the van 't Hoff equation p = iCRT, where p is osmotic pressure, i is the van 't Hoff factor, C is molar concentration, R is the gas constant, and T is temperature in Kelvin. In contrast, hydrodynamic pressure arises from fluid flow and mechanical forces, independent of solute concentration gradients.
Understanding Hydrodynamic Pressure
Hydrodynamic pressure refers to the pressure exerted by a fluid in motion, influenced by factors such as velocity, fluid density, and flow direction within pipelines or channels. It plays a critical role in systems involving fluid dynamics, impacting pump performance, pipe stress, and flow regulation. Unlike osmotic pressure, which originates from solute concentration differences across a semipermeable membrane, hydrodynamic pressure depends on physical movement and kinetic energy of the fluid.
Mechanisms of Osmotic Pressure Generation
Osmotic pressure arises from the difference in solute concentration across a semipermeable membrane, driving solvent molecules to move and balance solute levels without requiring external force. Mechanisms of osmotic pressure generation involve the selective permeability of membranes that allow solvent passage but restrict solute movement, creating a chemical potential gradient. Hydrodynamic pressure, in contrast, results from the physical force exerted by fluid flow or volume, not from solute concentration gradients.
Factors Affecting Hydrodynamic Pressure
Hydrodynamic pressure is influenced by factors such as fluid velocity, pipe diameter, and flow rate, which directly impact the kinetic energy of the moving fluid. In contrast, osmotic pressure depends on solute concentration differences across a semi-permeable membrane and is unrelated to fluid movement. Understanding hydrodynamic pressure requires analyzing fluid dynamics principles, including viscosity, turbulence, and resistance within the flow system.
Mathematical Expressions: Osmotic vs. Hydrodynamic Pressure
Osmotic pressure (p) is mathematically expressed as p = iMRT, where i is the van't Hoff factor, M is the molarity of the solute, R is the gas constant, and T is the absolute temperature, describing the pressure needed to stop solvent flow across a semipermeable membrane. Hydrodynamic pressure (P) is calculated using Bernoulli's equation or the Navier-Stokes equation depending on the context, typically involving fluid velocity, density, and gravitational effects, such as P = rgh for static fluids or P + 1/2rv2 + rgh = constant for flowing fluids. Both pressures play distinct roles: osmotic pressure governs solute-driven solvent migration, while hydrodynamic pressure influences fluid flow due to mechanical forces.
Biological Significance: Osmosis and Fluid Flow
Osmotic pressure drives water movement across semipermeable membranes, crucial for maintaining cell turgor and nutrient balance in biological systems. Hydrodynamic pressure governs bulk fluid flow within vascular systems, enabling blood circulation and nutrient transport. Together, these pressures regulate cellular homeostasis and organ function by coordinating water distribution and fluid dynamics.
Applications in Engineering and Medicine
Osmotic pressure is crucial in biomedical engineering for dialysis and drug delivery systems, as it governs the movement of fluids across semipermeable membranes, enabling selective solute transport. Hydrodynamic pressure plays a vital role in fluid mechanics and cardiovascular engineering, influencing blood flow dynamics and the design of pumps and vascular grafts. Understanding the interplay between osmotic and hydrodynamic pressures enhances the development of artificial organs and advanced filtration technologies in both medical and industrial applications.
Comparative Table: Osmotic vs. Hydrodynamic Pressure
Osmotic pressure is the force generated by the movement of solvent molecules through a semipermeable membrane to balance solute concentration, measured in atmospheres (atm) or Pascals (Pa). Hydrodynamic pressure refers to the pressure exerted by a fluid in motion, influenced by velocity and fluid density, typically measured in Pascals (Pa) or pounds per square inch (psi). Unlike osmotic pressure which is a colligative property depending on solute concentration, hydrodynamic pressure depends on flow dynamics and can vary with changes in fluid velocity and pipe diameter.
Conclusion: Key Differences and Practical Implications
Osmotic pressure arises from solute concentration differences across a semipermeable membrane, driving solvent flow to equalize concentrations, while hydrodynamic pressure results from fluid motion and external forces applied within a system. Key differences include osmotic pressure being a colligative property dependent on solute particles, whereas hydrodynamic pressure depends on fluid velocity and viscosity. In practical applications, understanding these pressures is crucial for designing water purification systems, biomedical devices, and industrial processes that rely on membrane separation or fluid transport.
Osmotic pressure Infographic
 libterm.com
libterm.com