Osmotic pressure is the force exerted by the movement of water through a semipermeable membrane from a region of low solute concentration to high solute concentration. It plays a crucial role in biological systems, affecting cell function and fluid balance. Discover how understanding osmotic pressure can impact your knowledge of chemistry and physiology by reading the rest of this article.
Table of Comparison
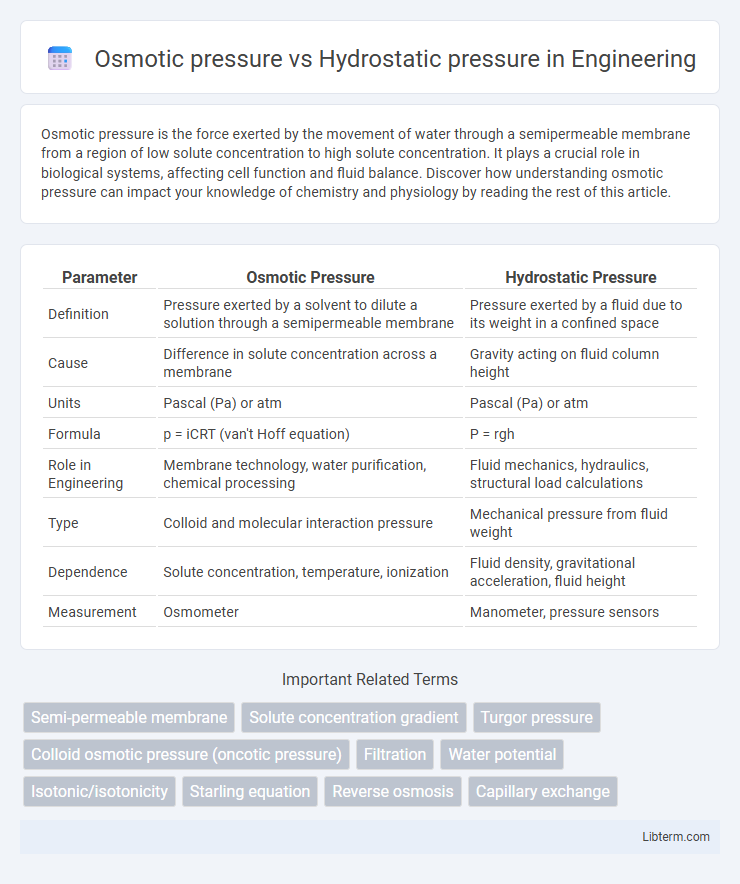
| Parameter | Osmotic Pressure | Hydrostatic Pressure |
|---|---|---|
| Definition | Pressure exerted by a solvent to dilute a solution through a semipermeable membrane | Pressure exerted by a fluid due to its weight in a confined space |
| Cause | Difference in solute concentration across a membrane | Gravity acting on fluid column height |
| Units | Pascal (Pa) or atm | Pascal (Pa) or atm |
| Formula | p = iCRT (van't Hoff equation) | P = rgh |
| Role in Engineering | Membrane technology, water purification, chemical processing | Fluid mechanics, hydraulics, structural load calculations |
| Type | Colloid and molecular interaction pressure | Mechanical pressure from fluid weight |
| Dependence | Solute concentration, temperature, ionization | Fluid density, gravitational acceleration, fluid height |
| Measurement | Osmometer | Manometer, pressure sensors |
Introduction to Osmotic and Hydrostatic Pressure
Osmotic pressure is the force exerted by the movement of water across a semipermeable membrane driven by solute concentration differences, crucial for maintaining cell turgor and fluid balance. Hydrostatic pressure is the pressure exerted by a fluid at equilibrium due to the force of gravity, significant in blood circulation and fluid movement in tissues. Both pressures play essential roles in biological systems, regulating fluid distribution and homeostasis.
Defining Osmotic Pressure
Osmotic pressure is the force exerted by the movement of water across a semipermeable membrane from a region of low solute concentration to high solute concentration, driven by osmosis. Hydrostatic pressure, in contrast, is the physical pressure exerted by a fluid in a confined space acting against the walls of its container or membrane. Understanding osmotic pressure is crucial in biological systems for maintaining cell turgor and in processes like dialysis and water purification.
Defining Hydrostatic Pressure
Hydrostatic pressure is the force exerted by a fluid at rest due to the weight of the fluid above a specific point, measured in pascals (Pa). It increases proportionally with the fluid's density and depth according to the equation \( P = \rho gh \), where \( \rho \) is density, \( g \) is gravitational acceleration, and \( h \) is height. Unlike osmotic pressure, which is driven by solute concentration gradients across a semipermeable membrane, hydrostatic pressure results from gravitational forces acting on the fluid column.
Key Differences Between Osmotic and Hydrostatic Pressure
Osmotic pressure is the force exerted by the movement of water across a semipermeable membrane due to solute concentration differences, measured in atmospheres (atm). Hydrostatic pressure is the physical pressure exerted by a fluid on the walls of its container or surrounding tissues, typically measured in millimeters of mercury (mmHg). Unlike hydrostatic pressure that depends on fluid volume and gravity, osmotic pressure is driven by solute concentration gradients and the tendency to equalize solute levels on both sides of the membrane.
Mechanisms of Osmotic Pressure in Biological Systems
Osmotic pressure in biological systems is driven by the movement of water across semipermeable membranes from areas of low solute concentration to high solute concentration, ensuring cellular homeostasis and nutrient balance. This process is crucial in maintaining cell turgor, regulating fluid exchange in capillaries, and facilitating nutrient absorption in tissues. Unlike hydrostatic pressure, which results from mechanical forces exerted by fluids, osmotic pressure relies on solute concentration gradients to control water dynamics in living organisms.
Role of Hydrostatic Pressure in Fluid Movement
Hydrostatic pressure plays a critical role in fluid movement by driving the flow of water and solutes across capillary walls from blood vessels into surrounding tissues. It counteracts osmotic pressure, which tends to pull water back into the capillaries, helping to maintain fluid balance between the vascular and interstitial compartments. This pressure difference is essential for nutrient delivery, waste removal, and overall tissue homeostasis.
Importance in Human Physiology
Osmotic pressure regulates water movement across cell membranes, maintaining cellular hydration and electrolyte balance essential for nerve impulse transmission and muscle function. Hydrostatic pressure drives blood flow within the cardiovascular system, ensuring efficient nutrient and oxygen delivery to tissues and removal of metabolic waste. The dynamic interplay between osmotic and hydrostatic pressures is crucial for maintaining fluid balance in organs such as the kidneys, preventing edema and supporting overall homeostasis.
Osmotic Pressure in Plant Cells
Osmotic pressure in plant cells is the force generated by the movement of water across a semipermeable membrane, driven by solute concentration differences inside the cell vacuole compared to the external environment. This pressure is crucial for maintaining cell turgor, enabling plants to stay rigid and upright by preventing wilting. Unlike hydrostatic pressure, which results from physical pressure exerted by fluids, osmotic pressure specifically regulates water uptake and distribution at the cellular level, influencing nutrient transport and plant growth.
Clinical Applications and Implications
Osmotic pressure plays a critical role in regulating fluid balance across capillary membranes, influencing intravenous fluid therapy by determining the movement of water between compartments, essential for managing dehydration and edema. Hydrostatic pressure drives the filtration of blood plasma through capillary walls, impacting conditions such as hypertension and heart failure by altering tissue perfusion and fluid accumulation. Clinical interventions often target these pressures to restore homeostasis, exemplified by the use of hypertonic solutions to adjust osmotic gradients or diuretics to reduce elevated hydrostatic pressure.
Summary: Comparing Osmotic and Hydrostatic Pressure
Osmotic pressure is the force exerted by solute concentration differences across a semipermeable membrane, driving water movement to balance solute levels. Hydrostatic pressure refers to the physical pressure exerted by a fluid within a closed system, such as blood pressure in vessels, pushing fluid outward. Comparing the two, osmotic pressure controls water flow based on solute gradients, while hydrostatic pressure results from fluid force, both crucial in regulating fluid exchange in biological and physical systems.
Osmotic pressure Infographic
 libterm.com
libterm.com