Crevice corrosion occurs in confined spaces where stagnant solutions accumulate, leading to localized metal deterioration. This form of corrosion is often found in crevices, gasket surfaces, and under deposits where oxygen access is limited, creating a corrosive environment. Explore the rest of this article to understand how you can identify, prevent, and manage crevice corrosion effectively.
Table of Comparison
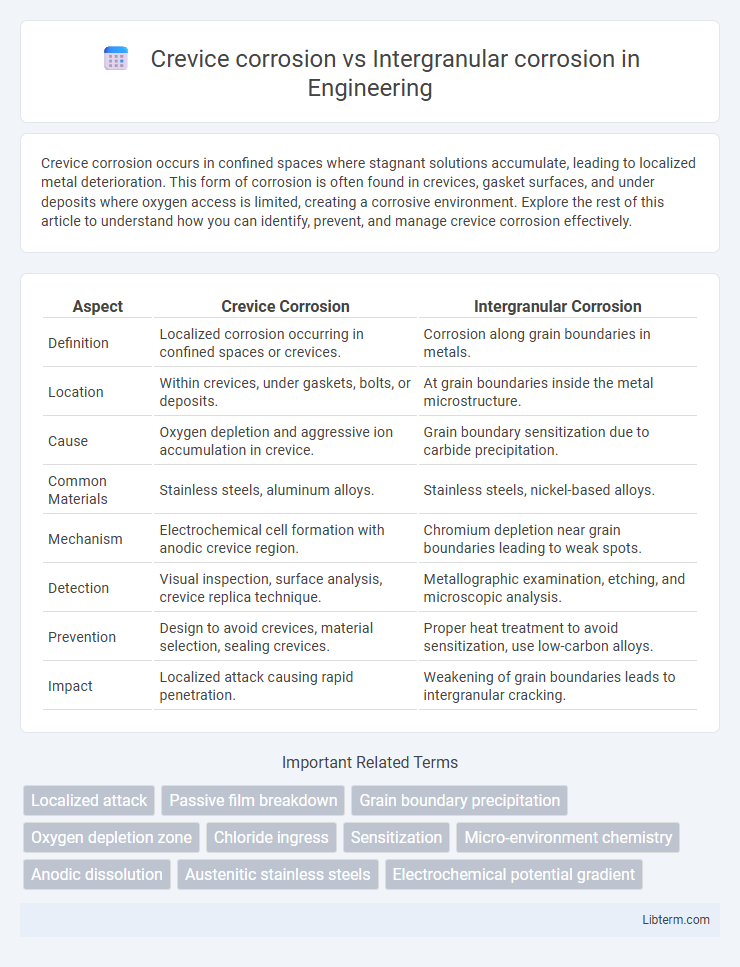
| Aspect | Crevice Corrosion | Intergranular Corrosion |
|---|---|---|
| Definition | Localized corrosion occurring in confined spaces or crevices. | Corrosion along grain boundaries in metals. |
| Location | Within crevices, under gaskets, bolts, or deposits. | At grain boundaries inside the metal microstructure. |
| Cause | Oxygen depletion and aggressive ion accumulation in crevice. | Grain boundary sensitization due to carbide precipitation. |
| Common Materials | Stainless steels, aluminum alloys. | Stainless steels, nickel-based alloys. |
| Mechanism | Electrochemical cell formation with anodic crevice region. | Chromium depletion near grain boundaries leading to weak spots. |
| Detection | Visual inspection, surface analysis, crevice replica technique. | Metallographic examination, etching, and microscopic analysis. |
| Prevention | Design to avoid crevices, material selection, sealing crevices. | Proper heat treatment to avoid sensitization, use low-carbon alloys. |
| Impact | Localized attack causing rapid penetration. | Weakening of grain boundaries leads to intergranular cracking. |
Introduction to Localized Corrosion
Localized corrosion targets specific areas of a metal surface, causing accelerated deterioration compared to uniform corrosion. Crevice corrosion occurs in confined spaces where stagnant solutions initiate corrosion, while intergranular corrosion attacks grain boundaries within the metal microstructure. Understanding these localized corrosion types is essential for preventing structural failures in critical industrial applications.
Defining Crevice Corrosion
Crevice corrosion occurs in confined spaces where stagnant solution causes localized chemical changes, leading to metal degradation, while intergranular corrosion attacks the grain boundaries of a metal due to chemical or structural inhomogeneities. Crevice corrosion typically forms in gaps, under deposits, or at gasket interfaces where oxygen depletion and chloride concentration accelerate corrosion. Understanding the electrochemical environment within crevices is essential for preventing material failure in marine, chemical processing, and aerospace industries.
Characteristics of Intergranular Corrosion
Intergranular corrosion primarily attacks the grain boundaries of a metal, causing a loss of cohesion between grains without significant surface penetration. This type of corrosion is often associated with sensitization in stainless steels, where chromium carbides precipitate at grain boundaries, depleting chromium and reducing corrosion resistance locally. Unlike crevice corrosion, which occurs in confined spaces, intergranular corrosion compromises the structural integrity internally, making it difficult to detect through surface examination.
Mechanisms Behind Crevice Corrosion
Crevice corrosion occurs in confined spaces where stagnant solution causes local changes in chemistry, leading to aggressive chloride accumulation and oxygen depletion that accelerate metal dissolution. This localized breakdown of the passive film happens due to differential aeration and acidification inside the crevice, creating an electrochemical cell that drives corrosion. In contrast, intergranular corrosion attacks grain boundaries due to precipitate formation or chromium depletion, without the reliance on trapped solution chemistry seen in crevice corrosion.
Causes of Intergranular Corrosion
Intergranular corrosion primarily arises from the precipitation of chromium carbides at grain boundaries, leading to chromium-depleted zones that lose their corrosion resistance. This phenomenon is often triggered by improper heat treatment or welding processes that expose stainless steel to temperatures between 450degC and 850degC. Unlike crevice corrosion, which is localized in shielded areas due to stagnant electrolyte, intergranular corrosion compromises the metal along grain boundaries, resulting in structural weakening.
Material Susceptibility: Comparison Table
Crevice corrosion primarily affects stainless steels and aluminum alloys exposed to stagnant chloride environments where oxygen depletion occurs, leading to localized attack within shielded areas. Intergranular corrosion targets grain boundaries in materials like sensitized austenitic stainless steels and nickel-based alloys, often caused by carbide precipitation or impurities that weaken grain boundary cohesion. The comparison table highlights crevice corrosion's reliance on geometric crevices and stagnant electrolytes, whereas intergranular corrosion depends on microstructural changes such as sensitization or segregation at grain boundaries, influencing their respective susceptibility profiles in specific alloy classes.
Environmental Factors Influencing Both Types
Crevice corrosion and intergranular corrosion are influenced by environmental factors such as oxygen concentration, temperature, and chloride ion presence. Low oxygen levels within crevices promote localized corrosion by creating differential aeration cells, while intergranular corrosion occurs along grain boundaries due to sensitization from thermal exposure in corrosive media. Both types accelerate in environments with high chloride concentrations and elevated temperatures, which disrupt protective oxide films and enhance metal susceptibility.
Detection and Identification Methods
Detection of crevice corrosion primarily involves visual inspection using borescopes and ultrasonic thickness measurements to identify localized metal loss within confined spaces. Intergranular corrosion is commonly detected through metallographic examination, including optical microscopy and scanning electron microscopy paired with energy-dispersive X-ray spectroscopy (EDS) to analyze grain boundary attack. Electrochemical techniques like potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) are applied to distinguish between these corrosion types by assessing differences in corrosion potentials and resistance.
Prevention and Mitigation Strategies
Crevice corrosion prevention involves design modifications to eliminate crevices and ensure uniform surface exposure, combined with the use of corrosion-resistant alloys like stainless steel or titanium and protective coatings. Intergranular corrosion mitigation relies on controlling material composition through stabilizing elements such as titanium or niobium, proper heat treatments to avoid sensitization, and maintaining clean welding practices to prevent grain boundary attack. Employing cathodic protection and corrosion inhibitors further enhances resistance against both crevice and intergranular corrosion in aggressive environments.
Industry Case Studies: Lessons Learned
Industry case studies reveal that crevice corrosion frequently occurs in confined spaces of marine and chemical processing equipment, where stagnant solutions accelerate localized attack, emphasizing the need for robust seal designs and material selection. Intergranular corrosion often arises in stainless steel weldments used in aerospace and power generation industries due to improper heat treatment, highlighting the critical importance of controlled thermal processes to preserve grain boundary integrity. Lessons learned stress implementation of advanced monitoring techniques and stringent QC protocols to mitigate both corrosion types and extend asset lifespan.
Crevice corrosion Infographic
 libterm.com
libterm.com