Uniform corrosion occurs evenly across a metal surface, gradually degrading the material's thickness and strength. This type of corrosion is predictable and easier to manage through protective coatings or corrosion inhibitors. Explore the rest of the article to understand how you can effectively prevent uniform corrosion and extend the lifespan of your metal structures.
Table of Comparison
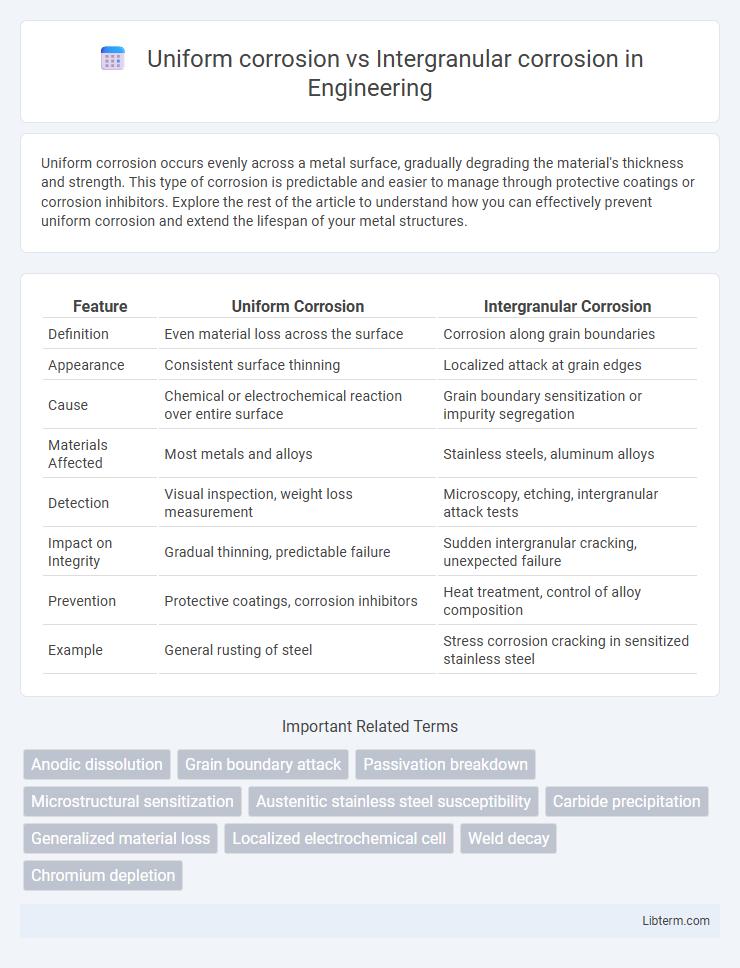
| Feature | Uniform Corrosion | Intergranular Corrosion |
|---|---|---|
| Definition | Even material loss across the surface | Corrosion along grain boundaries |
| Appearance | Consistent surface thinning | Localized attack at grain edges |
| Cause | Chemical or electrochemical reaction over entire surface | Grain boundary sensitization or impurity segregation |
| Materials Affected | Most metals and alloys | Stainless steels, aluminum alloys |
| Detection | Visual inspection, weight loss measurement | Microscopy, etching, intergranular attack tests |
| Impact on Integrity | Gradual thinning, predictable failure | Sudden intergranular cracking, unexpected failure |
| Prevention | Protective coatings, corrosion inhibitors | Heat treatment, control of alloy composition |
| Example | General rusting of steel | Stress corrosion cracking in sensitized stainless steel |
Introduction to Corrosion Types
Uniform corrosion occurs evenly across the entire surface of a metal, leading to a consistent material loss that is predictable and easier to monitor. Intergranular corrosion targets the grain boundaries within a metal's microstructure, causing localized weakening along these boundaries and often resulting in structural failures. Understanding the distinct mechanisms of uniform and intergranular corrosion is essential for selecting appropriate materials and corrosion protection methods in engineering applications.
What is Uniform Corrosion?
Uniform corrosion occurs evenly across the entire surface of a metal, resulting in consistent material loss and thickness reduction. This type of corrosion is typically caused by a chemical or electrochemical reaction with the surrounding environment, such as exposure to moisture, acids, or salts. In contrast to intergranular corrosion, uniform corrosion affects the surface uniformly without preferential attack along grain boundaries.
What is Intergranular Corrosion?
Intergranular corrosion occurs when the boundaries between metal grains corrode preferentially, compromising the structural integrity of alloys, especially stainless steels. Unlike uniform corrosion, which affects the entire surface evenly, intergranular corrosion attacks the grain boundaries due to chemical compositions or heat treatments that cause localized depletion of protective elements like chromium. This type of corrosion is critical in high-stress applications, as it can lead to unexpected material failure despite an apparently intact surface.
Mechanisms Behind Uniform Corrosion
Uniform corrosion occurs when metal surfaces corrode at a consistent rate due to an even electrochemical reaction, typically involving the oxidation of the metal and reduction of oxygen in the presence of an electrolyte. This process results from the homogeneous distribution of corrosive agents and environmental factors, such as humidity and temperature, leading to a stable corrosion potential across the entire metal surface. Unlike intergranular corrosion that attacks grain boundaries, uniform corrosion affects the bulk of the material, causing predictable material loss and allowing for easier monitoring and maintenance planning.
Mechanisms Behind Intergranular Corrosion
Intergranular corrosion occurs along the grain boundaries of a metal, caused by preferential attack at areas with chemical or structural differences compared to the grain interiors, often due to chromium carbide precipitation in stainless steels. This leads to the depletion of corrosion-resistant elements near the grain boundaries, creating anodic zones susceptible to corrosive environments. Unlike uniform corrosion, which evenly affects the entire surface, intergranular corrosion compromises the metal's integrity internally, making it difficult to detect until significant damage occurs.
Key Differences: Uniform vs Intergranular Corrosion
Uniform corrosion affects the entire surface of a metal evenly, resulting in consistent material loss and reduced thickness over time, commonly observed in steel exposed to acidic or saline environments. Intergranular corrosion targets the grain boundaries of a metal, often due to chromium depletion in stainless steels, causing weakened structural integrity along those boundaries without significant surface damage. Understanding these key differences is crucial for selecting appropriate corrosion prevention techniques and materials in industrial applications.
Materials Susceptible to Each Corrosion Type
Carbon steels and aluminum alloys commonly experience uniform corrosion due to their widespread use and exposure to corrosive environments. Intergranular corrosion primarily affects stainless steels, particularly those with improper heat treatment or sensitization, such as 304 and 316 grades. Nickel-based superalloys and certain high-strength aluminum alloys also show susceptibility to intergranular attack at grain boundaries where precipitates or chromium depletion occurs.
Detection and Identification Methods
Uniform corrosion manifests as consistent material loss across the entire surface, easily detected through visual inspection and weight loss measurements, while intergranular corrosion occurs along grain boundaries, requiring more advanced techniques such as metallographic examination, microscopy, and electrochemical testing for identification. Non-destructive evaluation methods like ultrasonic testing and eddy current testing assist in detecting subsurface intergranular corrosion damage that might not be visually apparent. Chemical analysis techniques, including X-ray diffraction (XRD) and energy-dispersive X-ray spectroscopy (EDS), further aid in characterizing corrosion products and distinguishing between uniform and intergranular corrosion mechanisms.
Prevention and Control Strategies
Uniform corrosion prevention relies on protective coatings, corrosion inhibitors, and cathodic protection to create a barrier against environmental exposure. Intergranular corrosion control focuses on material selection such as low-carbon or stabilized stainless steels, heat treatments like solution annealing, and minimizing sensitization by controlling cooling rates. Regular inspection and maintenance are critical for both types to detect early signs and apply remedial measures promptly.
Industry Examples and Practical Implications
Uniform corrosion occurs evenly across metal surfaces, commonly affecting pipelines and storage tanks in the oil and gas industry, leading to predictable material loss and straightforward maintenance. Intergranular corrosion targets grain boundaries, frequently impacting stainless steel components in chemical processing plants, causing localized weakening and unexpected failures. Understanding these corrosion types guides industry professionals in selecting appropriate materials and implementing targeted inspection protocols to enhance safety and longevity.
Uniform corrosion Infographic
 libterm.com
libterm.com