An isothermal line represents a continuous curve on a map that connects points of equal temperature at a specific time, crucial for understanding weather patterns and climate zones. These lines help meteorologists predict temperature changes and visualize thermal distribution across regions. Explore the rest of the article to uncover how isothermal lines influence your daily weather forecasting.
Table of Comparison
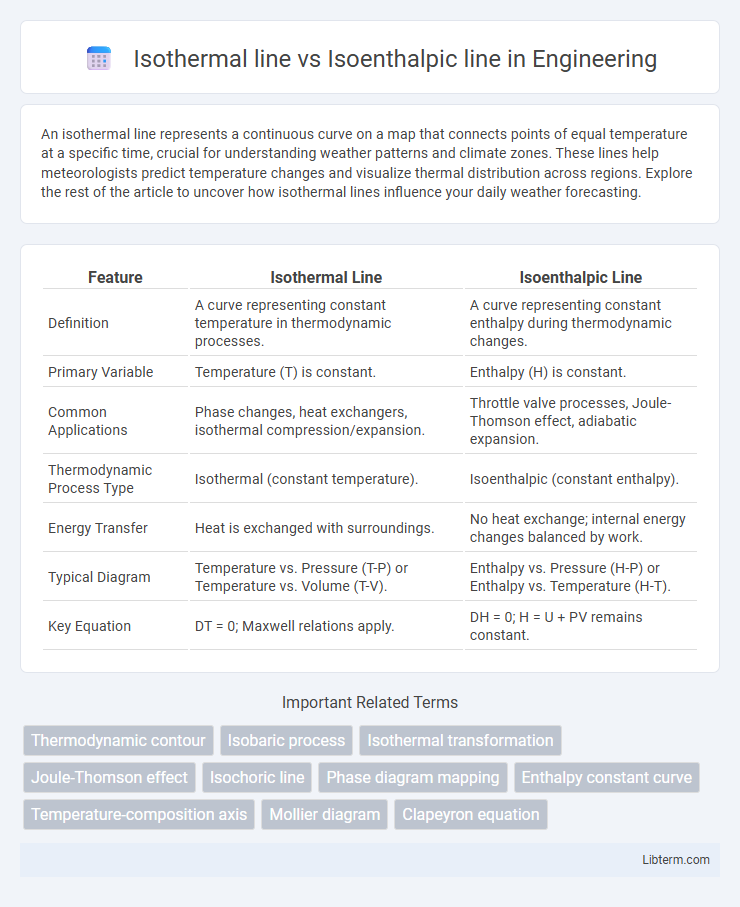
| Feature | Isothermal Line | Isoenthalpic Line |
|---|---|---|
| Definition | A curve representing constant temperature in thermodynamic processes. | A curve representing constant enthalpy during thermodynamic changes. |
| Primary Variable | Temperature (T) is constant. | Enthalpy (H) is constant. |
| Common Applications | Phase changes, heat exchangers, isothermal compression/expansion. | Throttle valve processes, Joule-Thomson effect, adiabatic expansion. |
| Thermodynamic Process Type | Isothermal (constant temperature). | Isoenthalpic (constant enthalpy). |
| Energy Transfer | Heat is exchanged with surroundings. | No heat exchange; internal energy changes balanced by work. |
| Typical Diagram | Temperature vs. Pressure (T-P) or Temperature vs. Volume (T-V). | Enthalpy vs. Pressure (H-P) or Enthalpy vs. Temperature (H-T). |
| Key Equation | DT = 0; Maxwell relations apply. | DH = 0; H = U + PV remains constant. |
Introduction to Isothermal and Isoenthalpic Lines
Isothermal lines represent states of constant temperature in thermodynamic systems, widely used in phase diagrams to analyze temperature-dependent behavior. Isoenthalpic lines depict conditions of constant enthalpy, crucial for understanding processes like throttling and expansion in thermodynamic cycles. Both lines serve as fundamental tools in thermal analysis, with isothermal lines emphasizing thermal equilibrium and isoenthalpic lines highlighting energy conservation during fluid flow.
Definition of Isothermal Line
The isothermal line represents a curve or path on a thermodynamic diagram along which temperature remains constant, regardless of changes in pressure or volume. It is commonly used in processes such as phase transitions and ideal gas behavior, where temperature equilibrium governs system states. In contrast, the isoenthalpic line depicts conditions where enthalpy is constant, emphasizing energy conservation rather than temperature stability.
Definition of Isoenthalpic Line
The isoenthalpic line represents a curve on a thermodynamic diagram where the enthalpy remains constant, corresponding to processes with no heat exchange and no work done, such as throttling or expansion through a valve. In contrast, the isothermal line depicts states with constant temperature, highlighting heat exchange to maintain thermal equilibrium. Understanding the isoenthalpic line is crucial for analyzing Joule-Thomson effects and gas expansion behavior in practical engineering applications.
Key Differences Between Isothermal and Isoenthalpic Lines
Isothermal lines represent constant temperature processes on thermodynamic charts, while isoenthalpic lines denote constant enthalpy processes. Key differences include the fact that isothermal processes involve heat exchange to maintain temperature, whereas isoenthalpic processes often occur without heat transfer, such as Joule-Thomson expansion. Thermodynamic property charts and phase diagrams use these lines to analyze energy transformations and fluid behavior under different thermal conditions.
Physical Significance in Thermodynamics
Isothermal lines represent constant temperature conditions on a thermodynamic diagram, indicating states where heat transfer occurs without temperature change, essential for analyzing processes like phase transitions. Isoenthalpic lines denote constant enthalpy, crucial for understanding expansions and throttling processes where enthalpy remains unchanged despite pressure or volume variations. The physical significance lies in isothermal lines characterizing equilibrium states at fixed temperatures, while isoenthalpic lines describe energy-conserving transformations critical for real-world applications such as refrigeration cycles and gas expansions.
Representation on P-V and T-S Diagrams
Isothermal lines represent constant temperature processes and appear as hyperbolic curves on P-V diagrams and horizontal or slightly curved lines on T-S diagrams. Isoenthalpic lines denote constant enthalpy processes, showing nearly vertical or slightly sloped lines on P-V diagrams and complex curves on T-S diagrams, reflecting enthalpy changes with pressure and entropy variations. The distinction between these lines assists in analyzing thermodynamic processes, with isothermal lines emphasizing temperature constancy and isoenthalpic lines highlighting enthalpy conservation.
Applications in Engineering and Physics
Isothermal lines represent constant temperature processes commonly used in thermodynamics for designing heat exchangers and refrigeration cycles, while isoenthalpic lines denote constant enthalpy processes critical in throttling valves and Joule-Thomson effect analysis. Engineers utilize isothermal lines for optimizing heat transfer in systems, whereas isoenthalpic lines aid in predicting fluid behavior during expansion or compression without heat exchange. In physics, isothermal processes model phase changes at equilibrium, and isoenthalpic lines facilitate understanding energy conservation in adiabatic flow systems.
Examples of Isothermal and Isoenthalpic Processes
Isothermal processes occur at constant temperature and are exemplified by the slow compression or expansion of an ideal gas in a piston-cylinder device, where heat exchange maintains the temperature despite volume changes. Isoenthalpic processes maintain constant enthalpy, demonstrated by the throttling of refrigerants in expansion valves or Joule-Thomson effect experiments where pressure drops without temperature change in ideal conditions. Practical applications include refrigeration cycles utilizing isothermal compression and expansion, and gas pipeline operations relying on isoenthalpic flow for pressure regulation.
Common Misconceptions and Clarifications
Isothermal lines represent constant temperature conditions on a thermodynamic chart, while isoenthalpic lines denote constant enthalpy, often misunderstood as interchangeable due to overlapping appearances on phase diagrams. A frequent misconception is that processes along an isenthalpic line maintain temperature, ignoring that enthalpy can remain constant even as temperature changes, especially in throttling or Joule-Thomson expansion processes. Clarifying these distinctions is crucial for accurate analysis in thermodynamics, ensuring proper application of properties like temperature stability in isothermal processes versus energy conservation in isoenthalpic transformations.
Summary: Choosing Between Isothermal and Isoenthalpic Analysis
Isothermal lines represent processes at constant temperature, crucial for understanding temperature-dependent phase changes and thermodynamic equilibrium. Isoenthalpic lines maintain constant enthalpy, essential for analyzing energy-conserving processes like throttling and expansion in turbines. Selecting between isothermal and isoenthalpic analysis depends on whether temperature control or enthalpy conservation dominates the thermodynamic process, impacting system design and efficiency assessments.
Isothermal line Infographic
 libterm.com
libterm.com