Lattice diffusion plays a crucial role in the movement of atoms within a crystalline solid, influencing material properties such as strength and conductivity. This process depends on factors like temperature, atomic size, and crystal structure, which determine the rate at which atoms migrate through the lattice. Explore the full article to understand how lattice diffusion impacts your materials and their performance.
Table of Comparison
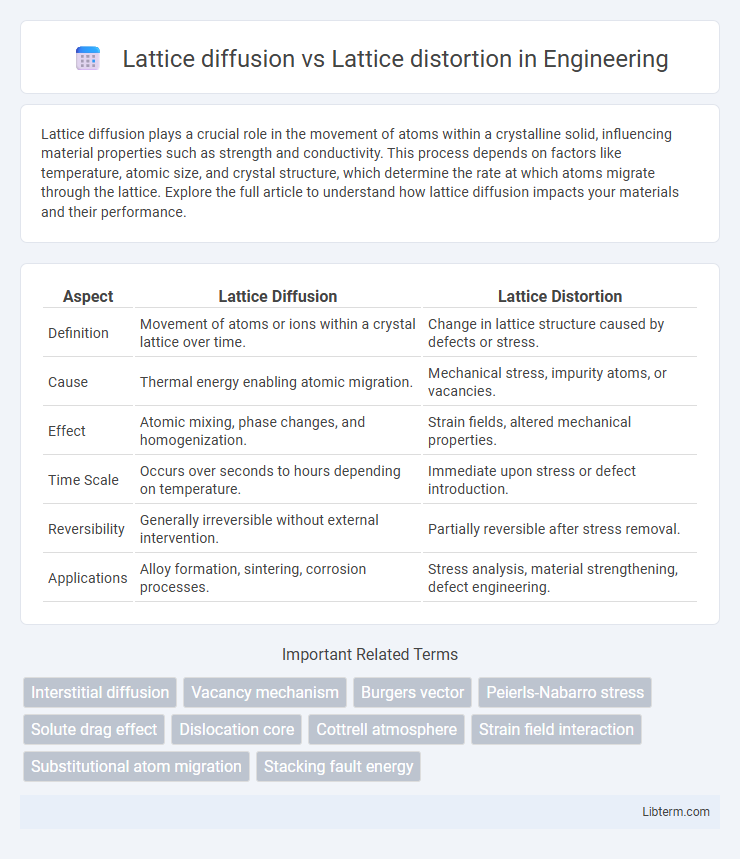
| Aspect | Lattice Diffusion | Lattice Distortion |
|---|---|---|
| Definition | Movement of atoms or ions within a crystal lattice over time. | Change in lattice structure caused by defects or stress. |
| Cause | Thermal energy enabling atomic migration. | Mechanical stress, impurity atoms, or vacancies. |
| Effect | Atomic mixing, phase changes, and homogenization. | Strain fields, altered mechanical properties. |
| Time Scale | Occurs over seconds to hours depending on temperature. | Immediate upon stress or defect introduction. |
| Reversibility | Generally irreversible without external intervention. | Partially reversible after stress removal. |
| Applications | Alloy formation, sintering, corrosion processes. | Stress analysis, material strengthening, defect engineering. |
Introduction to Lattice Diffusion and Lattice Distortion
Lattice diffusion refers to the movement of atoms or ions through the crystal lattice, driven by concentration gradients and thermal energy, playing a crucial role in processes like alloy formation and material aging. Lattice distortion occurs when the regular arrangement of atoms in a crystal is disrupted by defects, impurities, or external stress, affecting mechanical properties and electronic behavior. Understanding both phenomena is essential for optimizing material performance in applications such as semiconductor fabrication and metallurgy.
Fundamental Concepts of Lattice Structures
Lattice diffusion involves the movement of atoms or ions through the periodic arrangement of a crystal lattice, governed by vacancy or interstitial mechanisms that affect material properties like conductivity and strength. Lattice distortion refers to the deviation from perfect periodicity caused by defects such as substitutional or interstitial atoms, dislocations, or external stresses, which alter the local atomic arrangement and impact mechanical behavior. Understanding these fundamental concepts of lattice structures is crucial for tailoring material performance in applications like semiconductor devices and metallurgy.
What is Lattice Diffusion?
Lattice diffusion refers to the process by which atoms or ions move through the crystal lattice of a solid material, driven primarily by concentration gradients and temperature. This atomic migration affects material properties such as strength, conductivity, and phase transformations, playing a critical role in phenomena like alloying, sintering, and corrosion. Unlike lattice distortion, which involves the displacement and deformation of lattice atoms causing strain, lattice diffusion specifically describes the movement of particles within the lattice without permanent deformation.
What is Lattice Distortion?
Lattice distortion refers to the deviation of atoms from their ideal positions within a crystal lattice, often caused by defects, impurities, or external stresses. This distortion alters the local symmetry and can influence material properties such as electrical conductivity, mechanical strength, and diffusion rates. Unlike lattice diffusion, which involves the movement of atoms through the lattice, lattice distortion is a static or dynamic displacement affecting the structural integrity and behavior of the material.
Key Differences Between Lattice Diffusion and Distortion
Lattice diffusion refers to the process where atoms or ions move through a crystal lattice, facilitating material transport and affecting properties like conductivity and strength, while lattice distortion involves the displacement of atoms from their original positions, causing changes in the crystal structure and often resulting in strain or defects. Diffusion primarily impacts atomic mobility and is temperature-dependent, governed by activation energy and diffusion coefficients, whereas distortion alters the lattice symmetry, modifying mechanical and optical characteristics without significant atomic migration. Key differences lie in their mechanisms--diffusion is a dynamic mass transport phenomenon, while distortion is a static structural modification influencing material behavior under stress or external forces.
Mechanisms of Atomic Movement in Lattice Diffusion
Lattice diffusion involves the movement of atoms through vacancies or interstitial sites within the crystal lattice, driven by concentration gradients and thermal energy, enabling the redistribution of atoms over time. This atomic migration occurs via mechanisms such as vacancy diffusion where atoms exchange positions with adjacent vacancies, or interstitial diffusion where smaller atoms move through the spaces between larger atoms. In contrast, lattice distortion refers to the local displacement of atoms caused by defects or external stress, affecting the lattice structure but not involving long-range atomic transport.
Causes and Consequences of Lattice Distortion
Lattice distortion occurs due to the introduction of impurities, point defects, or dislocations that disrupt the regular atomic arrangement, leading to localized strain within the crystal structure. This strain alters the material's mechanical properties, such as hardness and ductility, by impeding dislocation motion and affecting electron mobility. In contrast, lattice diffusion involves the movement of atoms or vacancies through the crystal lattice, driven by concentration gradients or temperature, without necessarily causing permanent distortions to the lattice framework.
Effects on Material Properties: Diffusion vs Distortion
Lattice diffusion enhances atomic mobility, promoting processes like alloy homogenization and phase transformations, which improve material strength and ductility. Lattice distortion, caused by defects or solute atoms, generates internal stresses that can increase hardness and yield strength by hindering dislocation motion. The interplay between diffusion-driven microstructural evolution and distortion-induced mechanical reinforcement critically determines a material's performance under stress and temperature variations.
Applications and Significance in Materials Science
Lattice diffusion is crucial in processes such as alloy formation, sintering, and high-temperature corrosion resistance by enabling atomic movement within the crystal lattice, which affects material properties like strength and ductility. Lattice distortion, caused by defects or impurity atoms, significantly influences mechanical behavior, including hardness and yield strength, by impeding dislocation motion. Understanding both phenomena is essential for tailoring materials in aerospace, electronics, and metallurgy to optimize performance and durability.
Conclusion: Comparing Lattice Diffusion and Lattice Distortion
Lattice diffusion involves the movement of atoms or ions through a crystal lattice, significantly impacting material properties such as conductivity and strength. Lattice distortion refers to the displacement or deformation of atoms within the lattice, influencing mechanical behavior and defect formation. Comparing both, lattice diffusion primarily governs atomic mobility and mass transport, while lattice distortion affects structural integrity and resistance to mechanical stress.
Lattice diffusion Infographic
 libterm.com
libterm.com