An interstitial defect occurs when an extra atom occupies a space between the regular atomic sites in a crystal lattice, disrupting its perfect order. These defects can significantly affect the mechanical, electrical, and thermal properties of materials, often enhancing strength or altering conductivity. Dive into the rest of this article to understand how interstitial defects impact the performance of various materials and how you can control them in your applications.
Table of Comparison
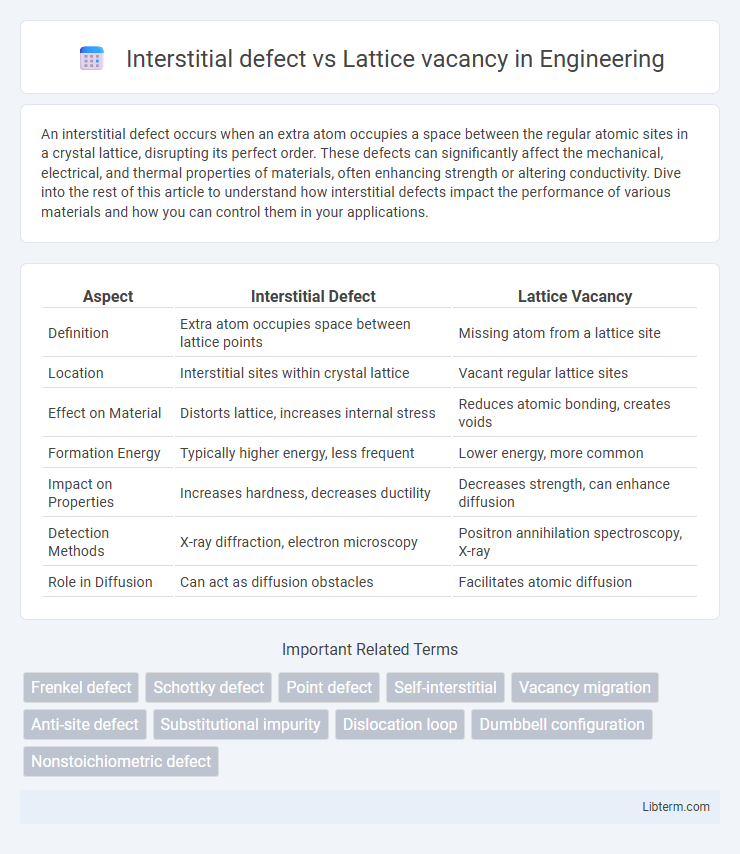
| Aspect | Interstitial Defect | Lattice Vacancy |
|---|---|---|
| Definition | Extra atom occupies space between lattice points | Missing atom from a lattice site |
| Location | Interstitial sites within crystal lattice | Vacant regular lattice sites |
| Effect on Material | Distorts lattice, increases internal stress | Reduces atomic bonding, creates voids |
| Formation Energy | Typically higher energy, less frequent | Lower energy, more common |
| Impact on Properties | Increases hardness, decreases ductility | Decreases strength, can enhance diffusion |
| Detection Methods | X-ray diffraction, electron microscopy | Positron annihilation spectroscopy, X-ray |
| Role in Diffusion | Can act as diffusion obstacles | Facilitates atomic diffusion |
Introduction to Crystal Defects
Interstitial defects occur when extra atoms occupy spaces between the regular lattice points in a crystal structure, causing local distortion and strain. Lattice vacancies are created by the absence of atoms at regular lattice positions, leading to empty sites that affect diffusion and mechanical properties. Both types of point defects critically influence the electrical, thermal, and mechanical behavior of crystalline materials by altering atomic arrangements and local bonding.
Defining Interstitial Defects
Interstitial defects occur when extra atoms occupy spaces between regular lattice sites, disrupting the crystal structure without removing any atoms. Lattice vacancies involve the absence of atoms from their normal lattice positions, creating empty sites within the crystal. Interstitial defects increase atomic density locally, affecting properties such as diffusion and mechanical strength more than lattice vacancies.
Understanding Lattice Vacancies
Lattice vacancies are atomic-scale defects where an atom is missing from its regular position in the crystal lattice, significantly affecting material properties such as diffusion, electrical conductivity, and mechanical strength. Unlike interstitial defects, where extra atoms occupy spaces between lattice sites, vacancies create empty lattice points that facilitate atom migration and influence defect dynamics. Understanding lattice vacancies is crucial for tailoring the performance of metals, semiconductors, and ceramics, especially in processes like annealing and doping.
Occurrence Mechanisms of Interstitial Defects
Interstitial defects form when atoms occupy positions between the regular lattice sites, often due to high-energy particle irradiation or rapid cooling that traps atoms in non-lattice sites. Lattice vacancies occur when atoms are missing from their normal lattice positions due to thermal vibrations or diffusion processes. The occurrence of interstitial defects is primarily driven by displacement events causing atoms to be forced into interstitial spaces, significantly affecting materials' mechanical and electrical properties.
Formation and Causes of Lattice Vacancies
Lattice vacancies form when atoms are missing from their regular positions in a crystal lattice due to thermal vibrations or external stresses, creating empty lattice sites that disrupt the material's atomic arrangement. Interstitial defects occur when extra atoms occupy spaces between the regular lattice sites, often caused by atom size mismatch or irradiation, leading to distortion in the crystal structure. The formation of lattice vacancies increases with temperature as atomic vibrations overcome bonding forces, and they play a critical role in diffusion and mechanical properties of metals and semiconductors.
Structural Differences: Interstitial vs Vacancy
Interstitial defects occur when extra atoms occupy spaces between the regular lattice sites, causing local lattice distortion due to atomic crowding. In contrast, lattice vacancies are formed by missing atoms at regular lattice points, creating empty atomic sites that disrupt the crystal's periodicity. The structural difference lies in interstitial defects adding atoms to the lattice interstices, while vacancies remove atoms from their designated lattice positions.
Impact on Material Properties
Interstitial defects introduce extra atoms into the crystal structure, causing lattice distortions that typically increase material hardness and strength but reduce electrical conductivity. Lattice vacancies, which are missing atoms in the crystal lattice, enhance atomic mobility and diffusion rates, often leading to decreased mechanical strength and increased material ductility. Both defect types critically influence thermal conductivity and corrosion resistance, with interstitials generally impeding heat flow and vacancies facilitating atomic migration processes.
Detection and Measurement Techniques
Interstitial defects and lattice vacancies in crystalline materials are detected and measured using techniques such as positron annihilation spectroscopy (PAS), which is highly sensitive to vacancies, and transmission electron microscopy (TEM), which can visualize interstitial defects at atomic resolution. X-ray diffraction (XRD) and Rutherford backscattering spectrometry (RBS) provide complementary data by analyzing distortions in lattice parameters caused by both vacancies and interstitials. Depth profiling and Doppler broadening spectroscopy refine the characterization of defect concentration and spatial distribution, enabling precise quantification for material performance evaluation.
Real-World Examples and Applications
Interstitial defects occur when extra atoms occupy spaces between regular lattice sites, as seen in steel where carbon atoms fill interstitial positions, enhancing hardness and strength. Lattice vacancies involve missing atoms in the crystal structure, influencing the diffusion process critical to semiconductor manufacturing, such as silicon wafer doping. Both defects play crucial roles in tailoring material properties for electronics, metallurgy, and nanotechnology applications.
Conclusion: Comparing Interstitial Defects and Lattice Vacancies
Interstitial defects introduce extra atoms into the crystal lattice, causing local distortion and increased atomic density, whereas lattice vacancies represent missing atoms, leading to decreased atomic density and potential diffusion pathways. The distinct impact on material properties depends on defect type; interstitial defects often enhance hardness and strength but reduce ductility, while lattice vacancies typically facilitate atomic diffusion and can influence electrical and thermal conductivity. Understanding the comparative roles of interstitial defects and lattice vacancies is crucial for tailoring materials' mechanical, electrical, and diffusion behaviors in semiconductor device fabrication and metallurgy.
Interstitial defect Infographic
 libterm.com
libterm.com