A substitutional atom replaces a host atom in a crystal lattice, altering the material's properties such as electrical conductivity, strength, or corrosion resistance. This atomic-level modification can tailor alloys and semiconductors for specific industrial applications. Explore the rest of the article to understand how substitutional atoms impact your material's performance and behavior.
Table of Comparison
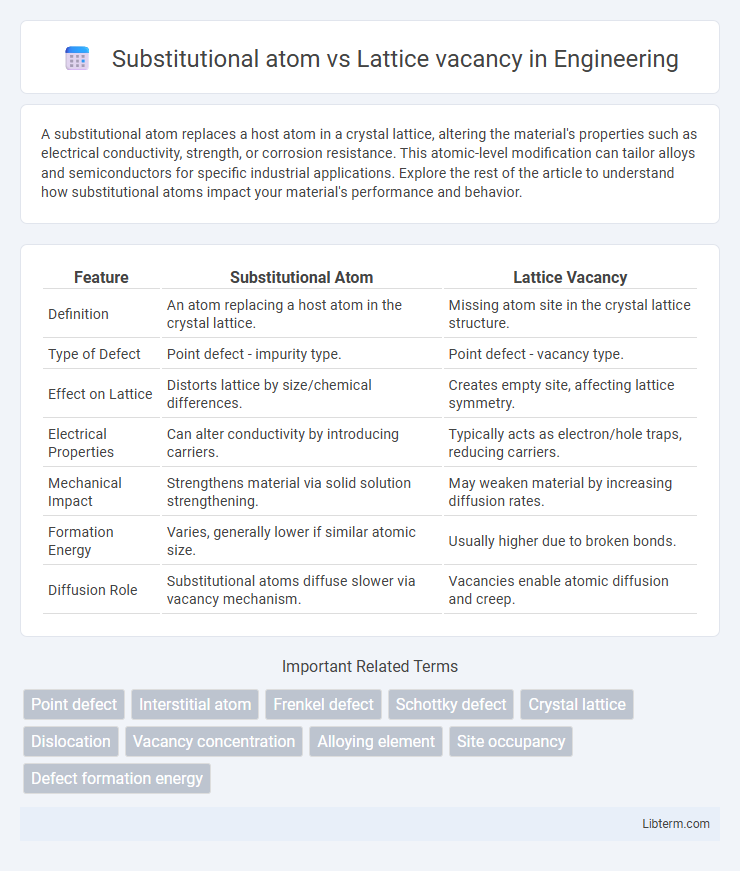
| Feature | Substitutional Atom | Lattice Vacancy |
|---|---|---|
| Definition | An atom replacing a host atom in the crystal lattice. | Missing atom site in the crystal lattice structure. |
| Type of Defect | Point defect - impurity type. | Point defect - vacancy type. |
| Effect on Lattice | Distorts lattice by size/chemical differences. | Creates empty site, affecting lattice symmetry. |
| Electrical Properties | Can alter conductivity by introducing carriers. | Typically acts as electron/hole traps, reducing carriers. |
| Mechanical Impact | Strengthens material via solid solution strengthening. | May weaken material by increasing diffusion rates. |
| Formation Energy | Varies, generally lower if similar atomic size. | Usually higher due to broken bonds. |
| Diffusion Role | Substitutional atoms diffuse slower via vacancy mechanism. | Vacancies enable atomic diffusion and creep. |
Introduction to Crystal Defects
Substitutional atoms are foreign atoms that replace host atoms at lattice sites within a crystal, altering its properties by introducing strain and affecting electrical conductivity. Lattice vacancies occur when atoms are missing from their regular lattice positions, creating empty sites that facilitate atomic diffusion and impact mechanical strength. Both substitutional atoms and lattice vacancies are fundamental point defects influencing the microstructure and behavior of crystalline materials.
Overview of Substitutional Atoms
Substitutional atoms are foreign atoms that replace host atoms at specific lattice sites within a crystalline solid, maintaining the overall crystal structure while altering its physical and chemical properties. These atoms differ in size, valence, or electronegativity from the host atoms, influencing material characteristics such as electrical conductivity, mechanical strength, and corrosion resistance. Unlike lattice vacancies, which are empty atomic sites disrupting the crystal lattice, substitutional atoms occupy regular lattice positions, enabling engineered modifications through alloying and doping in metals and semiconductors.
Understanding Lattice Vacancies
Lattice vacancies are point defects where atoms are missing from their regular positions in a crystal lattice, causing disruptions in the material's structural integrity and affecting properties like diffusion and electrical conductivity. Unlike substitutional atoms that replace host atoms with foreign atoms of different sizes, lattice vacancies create empty sites allowing atoms to move more freely, enhancing atomic diffusion rates. These vacancies play a critical role in processes such as creep, sintering, and the diffusion-controlled mechanisms that influence material strength and behavior under stress.
Formation Mechanisms of Substitutional Atoms
Substitutional atoms form when impurity atoms replace host atoms within a crystal lattice, typically occurring during processes such as alloying, diffusion, or high-temperature treatments. This mechanism relies on the similarity in atomic size and valence between the substitutional atom and the host atom to minimize lattice distortion and energy. In contrast, lattice vacancies are empty atomic sites created by thermal agitation or irradiation that disrupt the perfect lattice structure but do not involve atomic replacement.
Generation of Lattice Vacancies
Lattice vacancies are primarily generated through thermal activation, where atoms gain sufficient energy to leave their lattice positions, creating empty sites in the crystal structure. Substitutional atoms, in contrast, replace host atoms in the lattice without creating vacancies, typically introduced during alloying or doping processes. The generation of lattice vacancies significantly influences material properties like diffusion rates and mechanical strength by providing pathways for atomic movement.
Effects on Material Properties
Substitutional atoms introduce different elements into a crystal lattice, altering properties such as electrical conductivity, mechanical strength, and corrosion resistance by creating localized strain and disrupting electron flow. Lattice vacancies, or missing atoms in the crystal structure, increase atomic diffusion rates and can reduce density and mechanical strength by creating points of weakness. The balance between substitutional atoms and lattice vacancies significantly influences material performance, particularly in alloys and semiconductors.
Comparative Analysis: Substitutional Atom vs Lattice Vacancy
Substitutional atoms replace original atoms within a crystal lattice, altering local electronic and mechanical properties by introducing size and bonding differences. Lattice vacancies, on the other hand, represent missing atoms, creating voids that disrupt atomic coordination and influence diffusion rates and material strength. Comparative analysis shows substitutional atoms typically cause lattice distortion and possible alloy strengthening, whereas vacancies primarily affect diffusion kinetics and can lead to increased creep or vacancy-driven deformation.
Role in Diffusion Processes
Substitutional atoms alter diffusion rates by occupying lattice sites, creating localized strain fields that can either enhance or impede atomic movement depending on size and interaction energy. Lattice vacancies serve as primary diffusion vehicles, enabling atoms to hop into empty sites and facilitating self-diffusion and impurity diffusion in crystalline solids. The concentration and mobility of vacancies directly control diffusion coefficients, while substitutional atoms influence activation energy and diffusion pathways.
Detection and Characterization Techniques
Substitutional atoms and lattice vacancies are detected and characterized using techniques like Transmission Electron Microscopy (TEM) and Atom Probe Tomography (APT) that provide atomic-scale resolution and elemental identification. Positron Annihilation Spectroscopy (PAS) is particularly sensitive to lattice vacancies by measuring electron-positron annihilation characteristics, differentiating vacancy concentrations from substitutional atom distributions. X-ray Diffraction (XRD) and Secondary Ion Mass Spectrometry (SIMS) further differentiate substitutional atoms by analyzing lattice parameter changes and compositional depth profiles, respectively.
Applications and Significance in Materials Science
Substitutional atoms modify the electrical, mechanical, and thermal properties of alloys by replacing host atoms in the crystal lattice, enabling tailored material characteristics for semiconductors and high-strength alloys. Lattice vacancies facilitate diffusion processes essential for sintering, creep resistance, and phase transformations, impacting the durability and performance of metals and ceramics. Both defects are crucial for engineering material behavior in applications like microelectronics, catalysis, and structural components.
Substitutional atom Infographic
 libterm.com
libterm.com