Plating enhances the durability and appearance of metals by applying a thin layer of another metal onto the surface, often used to prevent corrosion and improve conductivity. Common plating materials include gold, silver, nickel, and chrome, each chosen for specific functional and aesthetic purposes. Explore the rest of this article to discover how plating can optimize your products and projects.
Table of Comparison
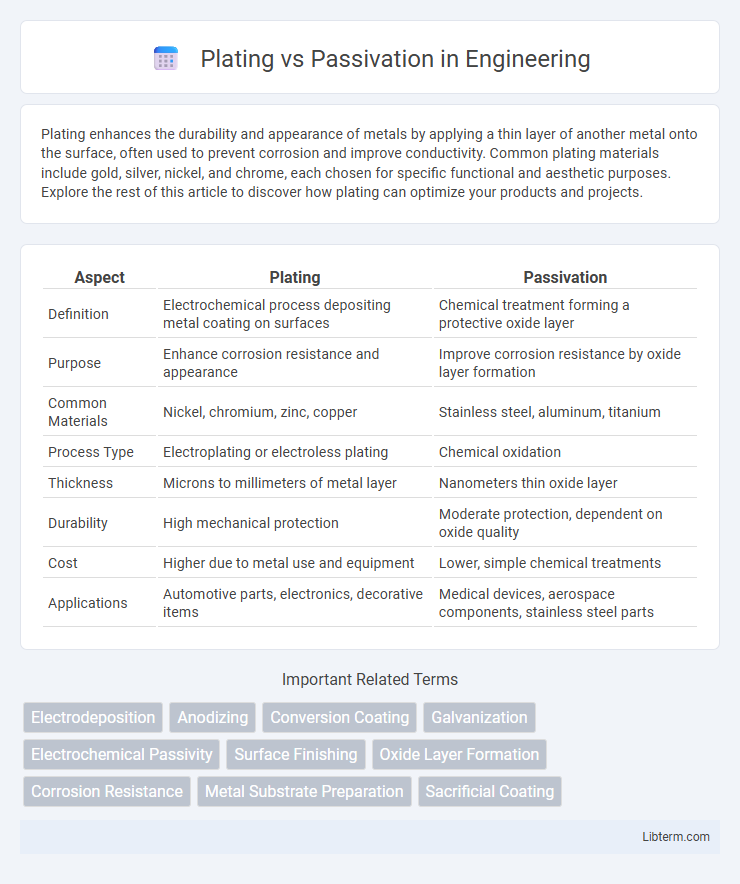
| Aspect | Plating | Passivation |
|---|---|---|
| Definition | Electrochemical process depositing metal coating on surfaces | Chemical treatment forming a protective oxide layer |
| Purpose | Enhance corrosion resistance and appearance | Improve corrosion resistance by oxide layer formation |
| Common Materials | Nickel, chromium, zinc, copper | Stainless steel, aluminum, titanium |
| Process Type | Electroplating or electroless plating | Chemical oxidation |
| Thickness | Microns to millimeters of metal layer | Nanometers thin oxide layer |
| Durability | High mechanical protection | Moderate protection, dependent on oxide quality |
| Cost | Higher due to metal use and equipment | Lower, simple chemical treatments |
| Applications | Automotive parts, electronics, decorative items | Medical devices, aerospace components, stainless steel parts |
Introduction to Plating and Passivation
Plating involves depositing a metal layer onto a surface to enhance corrosion resistance, wear resistance, or aesthetic appeal, commonly used with metals like nickel, chromium, and zinc. Passivation is a chemical treatment applied to metals such as stainless steel, creating a thin oxide layer that prevents rust and corrosion by making the surface less reactive. Both processes play essential roles in extending the durability and performance of metal components in various industrial applications.
What is Plating?
Plating is a surface finishing process where a thin layer of metal, such as nickel, chrome, or gold, is deposited onto a substrate to enhance its appearance, corrosion resistance, or conductivity. Common plating methods include electroplating, electroless plating, and immersion plating, each using chemical or electrical means to bond the metal layer. Plating is widely used in industries like electronics, automotive, and jewelry to improve product durability and performance.
What is Passivation?
Passivation is a chemical process that enhances the corrosion resistance of metal surfaces by creating a thin, inert oxide layer that protects against environmental damage. This protective film forms naturally on metals like stainless steel when exposed to oxidizing agents, preventing further oxidation and rust formation. Unlike plating, which involves applying a metal coating to improve surface properties, passivation improves durability without altering the metal's appearance or thickness.
Key Differences Between Plating and Passivation
Plating involves depositing a metal coating on a substrate to enhance corrosion resistance, aesthetics, or conductivity, while passivation is a chemical treatment that forms a protective oxide layer on metals like stainless steel to prevent corrosion. Plating adds a physical layer that can wear off over time, whereas passivation chemically stabilizes the metal surface without adding a distinct coating. The choice between plating and passivation depends on factors such as desired durability, appearance, and environmental resistance of the metal component.
Benefits of Plating Processes
Plating processes enhance metal surfaces by providing superior corrosion resistance, improved electrical conductivity, and increased wear resistance, making them ideal for industrial applications. Electroplating and electroless plating techniques enable uniform metal coatings that improve aesthetic appeal and extend component lifespan. These processes also facilitate solderability and provide a protective barrier against environmental factors, optimizing performance in automotive, aerospace, and electronics industries.
Advantages of Passivation Techniques
Passivation techniques enhance corrosion resistance by forming a protective oxide layer on metal surfaces, reducing oxidation and extending the lifespan of components compared to plating. These methods offer superior environmental resistance and maintain base metal integrity without adding thickness or altering dimensions. Passivation also improves surface cleanliness and purity, which is critical in industries requiring high hygiene standards such as aerospace, medical devices, and electronics.
Common Applications of Plating
Plating is widely used in industries such as automotive manufacturing, electronics, and jewelry for enhancing corrosion resistance, improving wear properties, and providing decorative finishes. Common plating materials include chromium, nickel, gold, and silver, each selected based on specific functional or aesthetic requirements. This surface treatment extends component lifespan, enhances conductivity in electronic devices, and adds value through attractive metallic coatings.
Typical Uses for Passivation
Passivation is commonly used in the semiconductor, medical device, and aerospace industries to enhance corrosion resistance and surface stability on stainless steel and other metal components. This electrochemical process forms a thin oxide layer that protects the metal from rust and contamination, extending the lifespan of critical parts. Typical applications include surgical instruments, electronic connectors, and precision aerospace hardware where durability and cleanliness are paramount.
Factors to Consider When Choosing Plating or Passivation
Selecting plating or passivation depends on factors like corrosion resistance requirements, the base material, and desired surface finish. Plating offers enhanced protection and improved conductivity through metals like nickel or chrome, while passivation primarily enhances corrosion resistance by forming a protective oxide layer on stainless steel. Cost implications, environmental impact, and application-specific durability also influence the decision between plating and passivation processes.
Conclusion: Plating vs Passivation for Metal Protection
Plating provides a durable, decorative, and corrosion-resistant coating by depositing a metal layer such as chromium or nickel, enhancing both aesthetics and surface hardness. Passivation improves metal corrosion resistance by forming a thin, inert oxide layer, often on stainless steel, without altering the surface appearance or thickness. For robust metal protection, plating is preferable in environments demanding physical barrier and wear resistance, while passivation excels in preserving corrosion resistance with minimal surface modification.
Plating Infographic
 libterm.com
libterm.com