Galvanic corrosion occurs when two different metals are electrically connected in the presence of an electrolyte, causing one metal to deteriorate faster than it would alone. This electrochemical process accelerates material degradation, especially in marine, automotive, and construction environments where moisture is common. Discover how to protect your structures and extend their lifespan by understanding key prevention strategies detailed in the rest of the article.
Table of Comparison
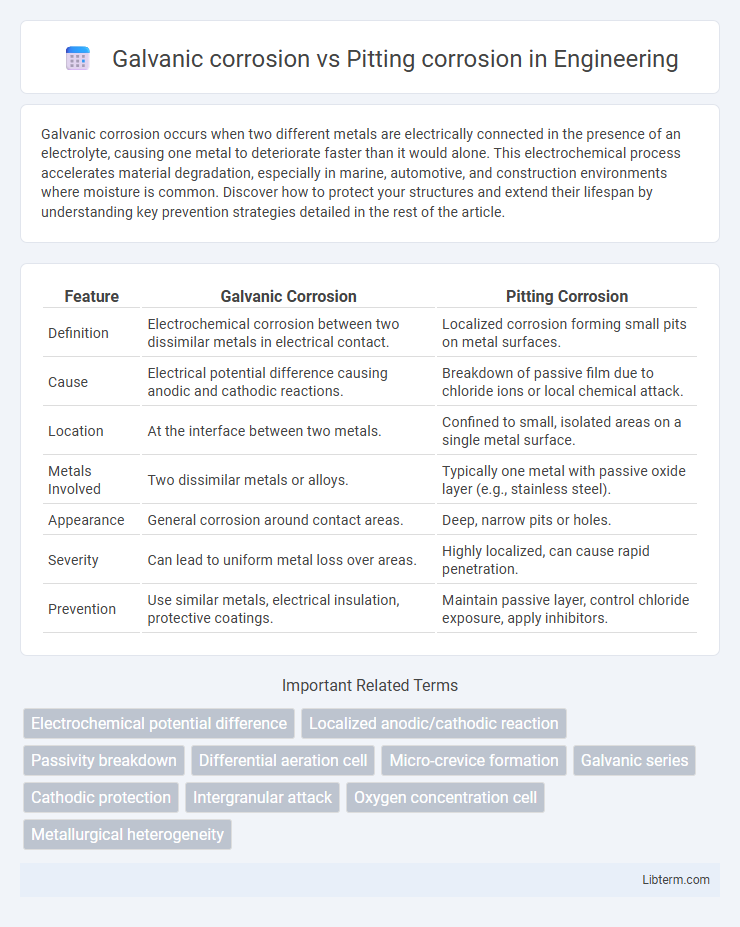
| Feature | Galvanic Corrosion | Pitting Corrosion |
|---|---|---|
| Definition | Electrochemical corrosion between two dissimilar metals in electrical contact. | Localized corrosion forming small pits on metal surfaces. |
| Cause | Electrical potential difference causing anodic and cathodic reactions. | Breakdown of passive film due to chloride ions or local chemical attack. |
| Location | At the interface between two metals. | Confined to small, isolated areas on a single metal surface. |
| Metals Involved | Two dissimilar metals or alloys. | Typically one metal with passive oxide layer (e.g., stainless steel). |
| Appearance | General corrosion around contact areas. | Deep, narrow pits or holes. |
| Severity | Can lead to uniform metal loss over areas. | Highly localized, can cause rapid penetration. |
| Prevention | Use similar metals, electrical insulation, protective coatings. | Maintain passive layer, control chloride exposure, apply inhibitors. |
Introduction to Corrosion Types
Galvanic corrosion occurs when two dissimilar metals are electrically connected in a corrosive electrolyte, causing accelerated metal loss on the anodic material due to electrochemical reactions. Pitting corrosion is a localized form of metal degradation characterized by small, often hard-to-detect pits or holes on a metal surface, typically initiated by the breakdown of the protective oxide layer in chloride-rich environments. Both types represent critical corrosion mechanisms that significantly impact the integrity and lifespan of metal structures in industrial and marine applications.
What is Galvanic Corrosion?
Galvanic corrosion occurs when two dissimilar metals are electrically connected in the presence of an electrolyte, causing one metal (the anode) to corrode faster while the other (the cathode) is protected. This electrochemical process accelerates degradation in metals such as steel and aluminum when paired improperly with more noble metals like copper or stainless steel. Understanding galvanic corrosion is crucial for designing metal systems in marine, industrial, and construction environments to prevent structural failures.
What is Pitting Corrosion?
Pitting corrosion is a localized form of corrosion that leads to the creation of small, deep cavities or pits on metal surfaces, often compromising structural integrity. It occurs when a passive film on metals like stainless steel is damaged, allowing aggressive ions such as chloride to penetrate and initiate the attack. Unlike galvanic corrosion, which involves an electrochemical reaction between two different metals, pitting corrosion targets a specific, isolated area, making it difficult to detect and control.
Causes of Galvanic Corrosion
Galvanic corrosion occurs when two dissimilar metals are electrically connected in the presence of an electrolyte, causing the more anodic metal to corrode faster. Causes of galvanic corrosion include differences in metal electrochemical potentials, the presence of an electrolyte such as water or moisture, and the size ratio of the anodic to cathodic metals. Understanding these factors is essential to prevent galvanic corrosion in metal structures and assemblies.
Causes of Pitting Corrosion
Pitting corrosion is primarily caused by localized breakdown of the passive oxide layer on metals such as stainless steel, often triggered by chloride ions in aggressive environments. Unlike galvanic corrosion, which occurs due to electrical potential differences between two different metals in contact, pitting corrosion initiates in small, discrete areas where the protective film is compromised, leading to deep, narrow pits. Factors such as stagnant conditions, low oxygen levels, and the presence of chlorides accelerate the creation of these anodic sites, making pitting a severe, concealed form of corrosion that can cause structural failures.
Key Differences Between Galvanic and Pitting Corrosion
Galvanic corrosion occurs when two dissimilar metals are electrically connected in a corrosive electrolyte, causing the more anodic metal to corrode faster, while pitting corrosion is a localized attack resulting in small, deep pits on a single metal surface. The key difference lies in galvanic corrosion involving two different metals and an electrochemical cell, whereas pitting corrosion affects only one metal and creates voids that penetrate the material. Galvanic corrosion typically progresses uniformly over large areas, whereas pitting corrosion is highly localized and often more difficult to detect and prevent.
Materials Most Susceptible to Each Corrosion Type
Galvanic corrosion primarily affects metals with different electrochemical potentials in direct contact, such as aluminum paired with copper, leading to accelerated degradation of the anodic material. Pitting corrosion commonly targets passive metals like stainless steel and aluminum alloys, where localized breakdown of the protective oxide layer causes deep, concentrated pits. Both corrosion types pose significant risks in marine and industrial environments, requiring careful material selection and protective measures.
Detection Methods for Galvanic and Pitting Corrosion
Galvanic corrosion detection methods include monitoring electrical potential differences between dissimilar metals using zero-resistance ammeter (ZRA) techniques and employing linear polarization resistance (LPR) to measure corrosion rates. Pitting corrosion is typically detected through visual inspection, scanning electron microscopy (SEM), and electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) that identify localized pit initiation and growth. Advanced methods like acoustic emission and X-ray computed tomography (CT) also provide detailed analysis of pit morphology and progression for accurate assessment.
Prevention and Mitigation Strategies
Galvanic corrosion prevention relies on electrically isolating dissimilar metals using coatings, insulating materials, or sacrificial anodes to interrupt the electrochemical cell. Pitting corrosion mitigation focuses on ensuring uniform protective film formation by maintaining proper alloy composition, avoiding crevices, and applying corrosion inhibitors to prevent localized breakdown. Both corrosion types benefit from routine inspection and environmental control, such as controlling moisture and chloride ion exposure, to extend material lifespan.
Conclusion: Choosing the Right Protection Method
Selecting the appropriate protection method hinges on understanding the distinct mechanisms of galvanic and pitting corrosion; galvanic corrosion requires materials with close electrochemical potentials or insulating barriers, while pitting corrosion demands robust coatings and inhibitors to prevent localized metal breakdown. Employing cathodic protection effectively counters galvanic corrosion by controlling electrode potentials, whereas corrosion-resistant alloys and surface treatments are essential to mitigate pitting in aggressive chloride environments. Tailoring protection strategies based on corrosion type and environmental conditions ensures optimal material longevity and structural integrity.
Galvanic corrosion Infographic
 libterm.com
libterm.com