Filtrate is the liquid that has passed through a filter, separating it from solid particles or impurities. It plays a crucial role in various industrial and laboratory processes, ensuring purity and clarity in the final product. Explore this article to understand how filtrate impacts your filtration applications and optimize your results.
Table of Comparison
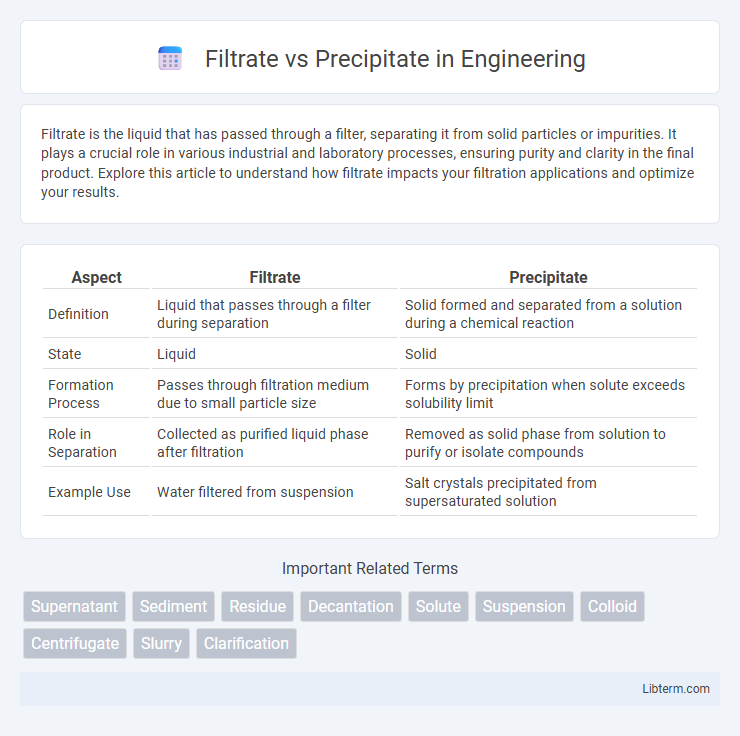
| Aspect | Filtrate | Precipitate |
|---|---|---|
| Definition | Liquid that passes through a filter during separation | Solid formed and separated from a solution during a chemical reaction |
| State | Liquid | Solid |
| Formation Process | Passes through filtration medium due to small particle size | Forms by precipitation when solute exceeds solubility limit |
| Role in Separation | Collected as purified liquid phase after filtration | Removed as solid phase from solution to purify or isolate compounds |
| Example Use | Water filtered from suspension | Salt crystals precipitated from supersaturated solution |
Introduction to Filtrate and Precipitate
Filtrate refers to the clear liquid that passes through a filter during the separation process, typically containing dissolved substances and smaller particles. Precipitate is the solid substance that forms and settles out of a solution when a chemical reaction causes certain dissolved ions to combine and become insoluble. Understanding the difference between filtrate and precipitate is essential in laboratory techniques such as filtration and crystallization.
Definitions: Filtrate Explained
Filtrate refers to the clear liquid that passes through a filter during the process of filtration, separating solid particles from a mixture. It contains dissolved substances and small particles that can pass through the filter medium. Understanding the concept of filtrate is essential in laboratory techniques, such as chemical analysis and purification processes.
Definitions: What is a Precipitate?
A precipitate is a solid substance that forms and separates from a liquid solution during a chemical reaction. It results from the insolubility of a compound, causing it to settle out of the solution as fine particles or crystals. Precipitates are commonly observed in reactions involving ionic compounds or changes in solubility conditions.
Formation Processes: How Filtrate and Precipitate Occur
Filtrate forms when a liquid passes through a filter medium, allowing dissolved substances and smaller particles to pass while retaining larger solids. Precipitate occurs when solutes chemically react or change solubility conditions, causing solid particles to form and separate out of a solution. The separation processes depend on physical filtration for filtrate and chemical precipitation for precipitate formation.
Filtration Techniques in Chemistry
Filtration techniques in chemistry involve separating solids from liquids using a porous barrier, where the filtrate is the clear liquid that passes through the filter, and the precipitate is the solid retained on the filter medium. Common filtration methods include gravity filtration for large particles, vacuum filtration for faster separation, and centrifugation to enhance sedimentation of fine precipitates. Optimizing filtration depends on particle size, solvent properties, and desired purity of the filtrate and precipitate for analytical or preparative purposes.
Precipitation Reactions and Mechanisms
Precipitation reactions occur when two soluble salts react in aqueous solution to form an insoluble solid called the precipitate, which separates from the filtrate, the clear liquid remaining after filtration. The mechanism involves the nucleation and growth of solid particles as ions in solution exceed their solubility product (Ksp), causing them to aggregate and settle out. Understanding the dynamics of ionic concentration, solubility equilibrium, and particle aggregation is crucial in controlling precipitate formation in analytical and synthetic chemistry.
Key Differences Between Filtrate and Precipitate
Filtrate refers to the clear liquid obtained after a mixture passes through a filter, containing dissolved substances, while precipitate is the solid formed and separated from a solution during a chemical reaction. Filtrate is collected on the filtrate side, and precipitate accumulates on the filter paper or container. Key differences include physical state--filtrate is liquid, precipitate is solid--and their formation process, with filtrate resulting from separation and precipitate from chemical change.
Practical Applications in Industry and Labs
Filtrate and precipitate are critical in chemical processes, where filtrate refers to the liquid that passes through a filter, essential for separating soluble substances in pharmaceuticals and food production. Precipitate, the solid formed from a reaction, plays a key role in wastewater treatment and mineral extraction by isolating contaminants or valuable compounds. Mastery of filtration and precipitation techniques optimizes recovery, purity, and efficiency in laboratory analysis and large-scale industrial operations.
Common Examples in Laboratory Practices
Filtrate and precipitate are distinct components in laboratory separations, with filtrate being the liquid that passes through a filter and precipitate the solid formed during a reaction. Common examples include the separation of sand from water where sand is the precipitate and water is the filtrate, or in qualitative analysis of ions like barium sulfate precipitating from a barium chloride and sulfuric acid reaction while the remaining liquid is the filtrate. Filtration techniques are crucial in processes such as recrystallization, wastewater treatment, and air purification, highlighting the practical roles of filtrate and precipitate in experimental and industrial contexts.
Summary: Choosing Filtrate or Precipitate for Analysis
Filtrate contains dissolved substances remaining in the liquid after a separation process, making it ideal for analyzing soluble compounds, ions, or contaminants. Precipitate consists of solid particles formed and collected during a reaction, providing concentrated material for identifying insoluble compounds or characterizing crystal structures. Selecting filtrate or precipitate for analysis depends on the target analytes and the desired form of sample for accurate chemical or physical examination.
Filtrate Infographic
 libterm.com
libterm.com