Solid solution strengthening enhances a metal's mechanical properties by adding impurity atoms to the crystal lattice, which hinder dislocation motion and increase hardness and strength. This process can involve substitutional atoms replacing host atoms or interstitial atoms fitting into the spaces within the lattice, creating lattice distortions that impede deformation. Discover how solid solution strengthening can improve your materials' performance in the rest of this article.
Table of Comparison
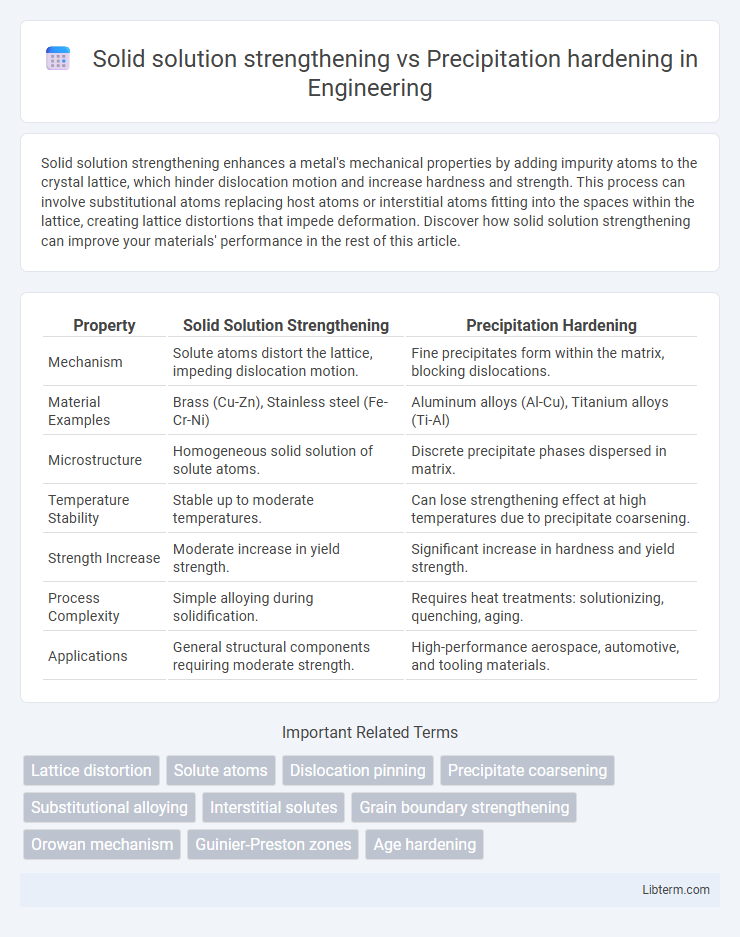
| Property | Solid Solution Strengthening | Precipitation Hardening |
|---|---|---|
| Mechanism | Solute atoms distort the lattice, impeding dislocation motion. | Fine precipitates form within the matrix, blocking dislocations. |
| Material Examples | Brass (Cu-Zn), Stainless steel (Fe-Cr-Ni) | Aluminum alloys (Al-Cu), Titanium alloys (Ti-Al) |
| Microstructure | Homogeneous solid solution of solute atoms. | Discrete precipitate phases dispersed in matrix. |
| Temperature Stability | Stable up to moderate temperatures. | Can lose strengthening effect at high temperatures due to precipitate coarsening. |
| Strength Increase | Moderate increase in yield strength. | Significant increase in hardness and yield strength. |
| Process Complexity | Simple alloying during solidification. | Requires heat treatments: solutionizing, quenching, aging. |
| Applications | General structural components requiring moderate strength. | High-performance aerospace, automotive, and tooling materials. |
Introduction to Strengthening Mechanisms in Metals
Solid solution strengthening increases metal strength by adding impurity atoms that distort the crystal lattice, hindering dislocation motion and enhancing yield strength. Precipitation hardening involves forming finely dispersed secondary phase particles that obstruct dislocation movement, significantly improving hardness and tensile strength. Both mechanisms are critical in metallurgy for tailoring mechanical properties through microstructural control.
Overview of Solid Solution Strengthening
Solid solution strengthening improves metal strength by dissolving alloying elements into the base metal, creating lattice distortions that impede dislocation movement. This method relies on substitutional or interstitial atoms differing in size or modulus from the host atoms, enhancing yield strength without forming separate phases. The effectiveness of solid solution strengthening depends on factors such as solute concentration, size mismatch, and elastic modulus differences between solute and solvent atoms.
Fundamentals of Precipitation Hardening
Precipitation hardening, also known as age hardening, involves the formation of fine, uniformly dispersed second-phase particles within a metal matrix, which obstruct dislocation motion and significantly increase strength. These precipitates nucleate and grow during controlled heat treatment processes that include solution treatment, quenching, and aging, resulting in enhanced mechanical properties compared to solid solution strengthening, which relies on lattice distortions caused by solute atoms. The effectiveness of precipitation hardening depends on factors such as precipitate size, distribution, coherence with the matrix, and the nature of the alloy system.
Atomic Mechanisms Behind Solid Solution Strengthening
Solid solution strengthening occurs when solute atoms distort the crystal lattice of the base metal, creating localized strain fields that impede dislocation motion and enhance material strength. These atomic-scale distortions increase resistance to slip by altering the energy landscape of dislocation movement. In contrast, precipitation hardening relies on finely distributed secondary phase particles obstructing dislocations, rather than lattice distortions caused by solute atoms.
Microstructural Changes in Precipitation Hardening
Precipitation hardening involves the formation of finely dispersed second-phase particles within the metal matrix that hinder dislocation motion, resulting in increased strength. Microstructural changes include nucleation, growth, and uniform distribution of precipitates such as intermetallic compounds or carbides, which create obstacles to slip and enhance hardness. In contrast, solid solution strengthening relies on atomic-scale distortions from solute atoms dispersed in the matrix, without forming distinct second-phase particles.
Comparative Effectiveness: Strength Gains Analyzed
Solid solution strengthening increases strength by dissolving alloying elements into the base metal, causing lattice distortions that hinder dislocation movement, typically yielding moderate strength improvements of up to 20-30%. Precipitation hardening enhances strength more significantly by forming fine, dispersed particles that obstruct dislocation slip, often resulting in strength gains exceeding 50% depending on the alloy system and aging conditions. The comparative effectiveness depends on factors such as alloy composition and processing, with precipitation hardening generally providing superior strength enhancement for high-performance applications.
Factors Influencing Strengthening Efficiency
The strengthening efficiency of solid solution strengthening depends on factors such as the size difference between solute and solvent atoms, the concentration of solute atoms, and the modulus mismatch, which create lattice distortions that impede dislocation motion. In precipitation hardening, the size, distribution, coherency, and volume fraction of precipitates critically influence the obstacle strength against dislocation glide, enhancing hardness and strength. Temperature and aging time also play vital roles by affecting solute diffusion and precipitate growth dynamics, thereby determining the overall effectiveness of precipitation hardening.
Applications in Industry: Where Each Method Excels
Solid solution strengthening is widely used in industries requiring improved corrosion resistance and moderate strength enhancement, such as aerospace and automotive sectors, where alloys like stainless steel and aluminum use substitutional or interstitial atoms to increase durability. Precipitation hardening excels in applications demanding high strength-to-weight ratios and enhanced mechanical properties, common in aerospace, military equipment, and high-performance sports gear, utilizing controlled heat treatments to form fine particles that impede dislocation motion. Both methods optimize alloy performance, but precipitation hardening offers superior strength increases ideal for critical load-bearing components, while solid solution strengthening provides balanced improvements beneficial for complex forming and welding processes.
Limitations and Challenges of Each Technique
Solid solution strengthening faces limitations such as lower achievable strength compared to other methods and potential decreases in ductility due to solute atom distortion in the crystal lattice. Precipitation hardening challenges include complex heat treatment requirements, sensitivity to aging time and temperature, and the risk of over-aging that reduces mechanical properties. Both techniques require precise control over alloy composition and processing parameters to optimize performance and avoid detrimental effects like brittleness or phase instability.
Future Trends in Alloy Strengthening Technologies
Future trends in alloy strengthening technologies emphasize the integration of solid solution strengthening with advanced precipitation hardening to achieve superior mechanical properties and enhanced thermal stability. Emerging research explores nanoscale precipitate engineering combined with high-entropy alloys to optimize strength and ductility simultaneously. Innovations in computational materials science and additive manufacturing enable precise control over alloy composition and microstructure, driving the next generation of high-performance structural materials.
Solid solution strengthening Infographic
 libterm.com
libterm.com