Cohesion is the quality that makes a text clear and unified by linking sentences and ideas smoothly through consistent vocabulary and grammatical structures. It enhances readability and ensures that your message flows logically, helping readers follow your arguments without confusion. Discover more about effective cohesion techniques to strengthen your writing in the rest of this article.
Table of Comparison
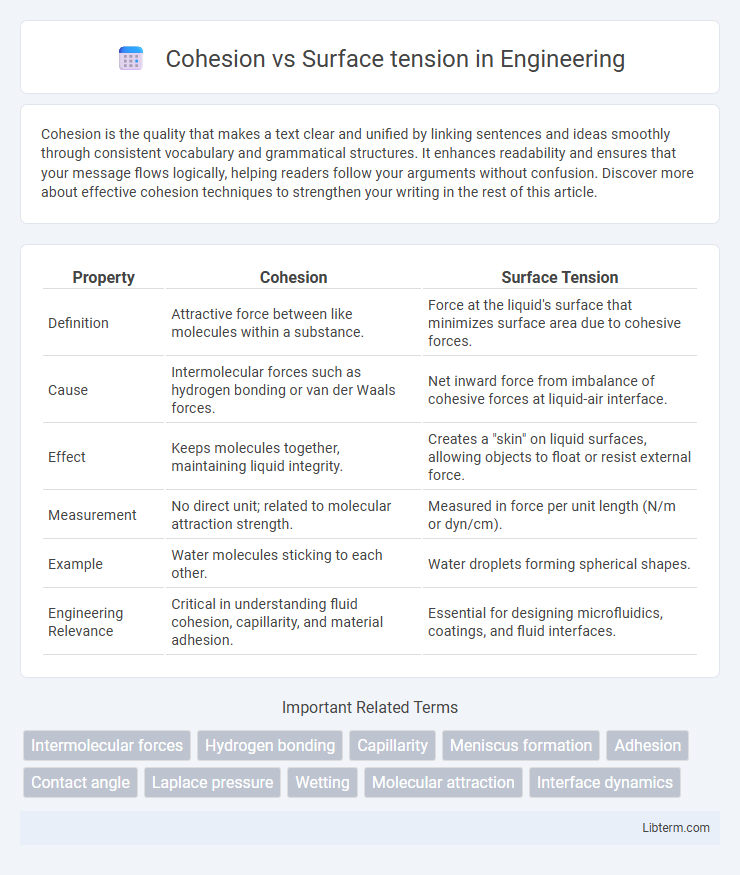
| Property | Cohesion | Surface Tension |
|---|---|---|
| Definition | Attractive force between like molecules within a substance. | Force at the liquid's surface that minimizes surface area due to cohesive forces. |
| Cause | Intermolecular forces such as hydrogen bonding or van der Waals forces. | Net inward force from imbalance of cohesive forces at liquid-air interface. |
| Effect | Keeps molecules together, maintaining liquid integrity. | Creates a "skin" on liquid surfaces, allowing objects to float or resist external force. |
| Measurement | No direct unit; related to molecular attraction strength. | Measured in force per unit length (N/m or dyn/cm). |
| Example | Water molecules sticking to each other. | Water droplets forming spherical shapes. |
| Engineering Relevance | Critical in understanding fluid cohesion, capillarity, and material adhesion. | Essential for designing microfluidics, coatings, and fluid interfaces. |
Understanding Cohesion and Surface Tension
Cohesion refers to the intermolecular attraction between similar molecules, such as water molecules bonding through hydrogen bonds, which helps maintain the fluid's integrity. Surface tension is a direct result of cohesive forces at the liquid's surface, creating a "skin-like" barrier that resists external force and allows objects like insects to rest on water without sinking. Understanding cohesion and surface tension is essential in fields such as biology, chemistry, and physics, where these phenomena influence processes like capillary action and droplet formation.
Defining Cohesion: Molecular Attraction Explained
Cohesion refers to the intermolecular attraction between like molecules, particularly evident in liquids such as water where hydrogen bonds create strong molecular connections. This molecular attraction enables substances to maintain structural integrity and resist external forces, differentiating cohesion from surface tension, which is the elastic tendency at a liquid's surface caused by cohesive forces. Understanding cohesion at the molecular level explains phenomena like water droplets forming spheres and the movement of water through plant xylem vessels.
What is Surface Tension?
Surface tension is the elastic-like force existing at the surface of a liquid, caused by the cohesive attraction between liquid molecules. This phenomenon allows liquids to resist external force, creating a "skin" that enables small objects to float or insects to walk on water. Surface tension is measured in force per unit length, typically in newtons per meter (N/m).
The Science Behind Cohesion
Cohesion refers to the intermolecular forces that hold like molecules together, primarily hydrogen bonding in water, creating a unified and stable structure. This molecular attraction enables phenomena such as water droplets forming and plant water transport through xylem vessels via capillary action. Understanding cohesion explains how molecules resist separation, contrasting with surface tension, which arises from the imbalance of cohesive forces at a liquid's surface.
Mechanisms of Surface Tension
Surface tension arises from cohesive forces between liquid molecules at the interface, where molecules experience a net inward force due to stronger attraction to neighboring molecules rather than air. These cohesive intermolecular forces, primarily hydrogen bonding in water, create a minimized surface area, resulting in a contractive effect on the liquid's surface. Unlike cohesion, which refers to the overall attraction between like molecules throughout the liquid, surface tension specifically describes the elastic-like behavior of the liquid surface caused by these unbalanced cohesive forces.
Key Differences: Cohesion vs. Surface Tension
Cohesion refers to the attraction between similar molecules, such as water molecules bonding through hydrogen bonds, which contributes to the structural integrity of liquids. Surface tension arises from cohesive forces at the liquid's surface, creating a "skin" effect that resists external force and allows objects like insects to walk on water. Key differences include cohesion acting throughout the entire liquid volume, whereas surface tension specifically affects the liquid's surface layer.
Real-Life Examples of Cohesion
Cohesion refers to the attractive force between molecules of the same substance, such as water molecules sticking together to form droplets on a window or enabling the water column to move upward in plants through capillary action. Surface tension, a result of cohesion, allows insects like water striders to walk on water surfaces without sinking. These phenomena demonstrate the critical role of hydrogen bonding in water's cohesive properties that support various biological and environmental processes.
Everyday Applications of Surface Tension
Surface tension enables water droplets to form beads on surfaces, allowing for efficient rain runoff on leaves and waterproof clothing. It plays a crucial role in capillary action, which helps plants absorb water from the soil through tiny xylem vessels. This phenomenon also facilitates the functionality of detergents in cleaning by breaking surface tension, enabling better penetration into fabrics and surfaces.
Importance of Cohesion and Surface Tension in Nature
Cohesion enables water molecules to stick together, crucial for processes like transpiration in plants, where water travels upward against gravity through xylem vessels. Surface tension creates a protective barrier on water surfaces, allowing insects like water striders to walk on lakes without sinking and facilitating the formation of droplets. Both cohesion and surface tension maintain ecosystem stability by supporting water transport, habitat formation, and interactions among organisms.
Summary: Cohesion and Surface Tension Compared
Cohesion refers to the attractive force between like molecules, such as water molecules bonding through hydrogen bonds, which helps maintain the integrity of the liquid. Surface tension is the result of cohesive forces at the liquid's surface, creating a 'skin' that resists external force and allows objects to float or insects to walk on water. While cohesion acts throughout the liquid body, surface tension specifically manifests at the liquid-air interface due to unbalanced molecular forces.
Cohesion Infographic
 libterm.com
libterm.com