An isobaric process occurs when a thermodynamic system undergoes a change at constant pressure, resulting in heat transfer that changes the system's volume and temperature. This type of process is commonly observed in engines and various heating systems where the pressure remains steady while the internal energy varies. Discover more about how isobaric processes affect energy efficiency and real-world applications in the rest of this article.
Table of Comparison
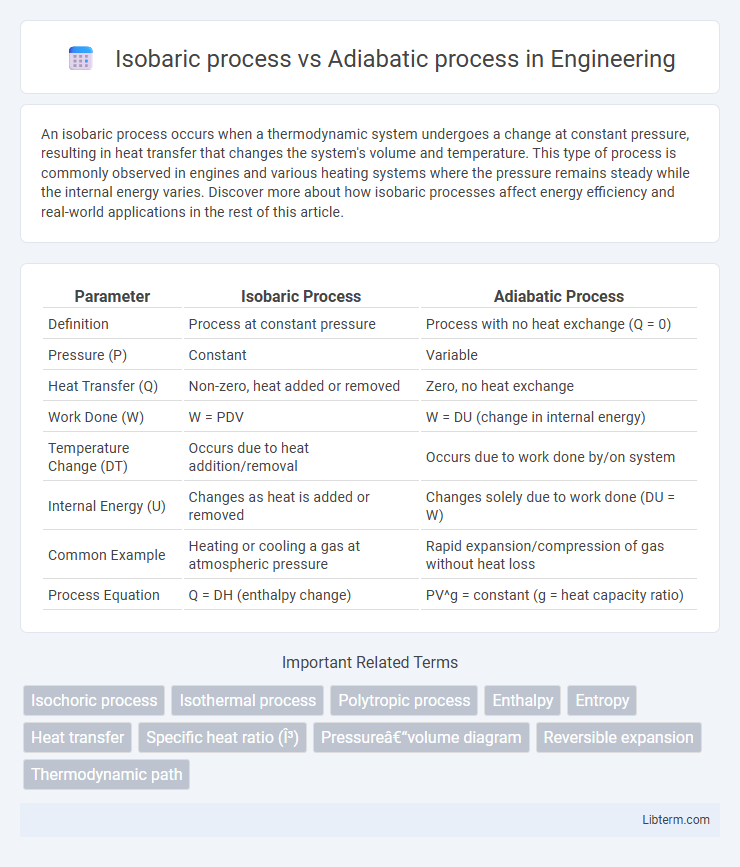
| Parameter | Isobaric Process | Adiabatic Process |
|---|---|---|
| Definition | Process at constant pressure | Process with no heat exchange (Q = 0) |
| Pressure (P) | Constant | Variable |
| Heat Transfer (Q) | Non-zero, heat added or removed | Zero, no heat exchange |
| Work Done (W) | W = PDV | W = DU (change in internal energy) |
| Temperature Change (DT) | Occurs due to heat addition/removal | Occurs due to work done by/on system |
| Internal Energy (U) | Changes as heat is added or removed | Changes solely due to work done (DU = W) |
| Common Example | Heating or cooling a gas at atmospheric pressure | Rapid expansion/compression of gas without heat loss |
| Process Equation | Q = DH (enthalpy change) | PV^g = constant (g = heat capacity ratio) |
Introduction to Isobaric and Adiabatic Processes
Isobaric processes occur at constant pressure, causing the system's volume and temperature to change while heat is exchanged with the surroundings. Adiabatic processes involve no heat transfer, leading to changes in pressure, volume, and temperature strictly due to work done by or on the system. Understanding these thermodynamic processes is essential for analyzing energy transfer in engines, compressors, and atmospheric phenomena.
Defining Isobaric Process
An isobaric process occurs at constant pressure, where the system's volume changes as heat is added or removed, leading to work being done by or on the system according to the equation W = PDV. In contrast, an adiabatic process involves no heat exchange with the surroundings, resulting in changes in pressure and temperature purely due to work interactions within the system. Understanding the distinction between isobaric and adiabatic processes is crucial in thermodynamics for analyzing energy transfer and system behavior under different constraints.
Defining Adiabatic Process
An adiabatic process is a thermodynamic transformation in which no heat is exchanged between the system and its surroundings, resulting in changes in pressure, volume, and temperature solely due to internal energy variations. Unlike an isobaric process, where pressure remains constant, adiabatic processes are characterized by rapid compression or expansion that prevents heat transfer, often described by the relation PV^g = constant for ideal gases. Understanding adiabatic behavior is crucial for applications in engines, compressors, and atmospheric science where thermal insulation impacts system dynamics.
Key Differences Between Isobaric and Adiabatic Processes
Isobaric processes occur at constant pressure while adiabatic processes involve no heat exchange with the surroundings. In isobaric processes, the volume changes to maintain constant pressure, whereas adiabatic processes experience changes in both pressure and volume without heat transfer. The key difference lies in heat interaction: isobaric processes allow heat exchange, but adiabatic processes rely solely on work done by or on the system for energy changes.
Thermodynamic Equations Involved
Isobaric processes maintain constant pressure, described by the equation \( Q = \Delta H = n C_p \Delta T \), where \( Q \) is heat added, \( \Delta H \) is enthalpy change, \( n \) moles of gas, \( C_p \) specific heat at constant pressure, and \( \Delta T \) temperature change. Adiabatic processes occur without heat exchange, governed by \( PV^\gamma = \text{constant} \) and \( TV^{\gamma -1} = \text{constant} \), where \( P \) is pressure, \( V \) volume, \( T \) temperature, and \( \gamma = C_p/C_v \) the heat capacity ratio. The first law of thermodynamics simplifies to \( \Delta U = W \) in adiabatic processes, highlighting the work done by or on the system without heat transfer.
Energy Transfer and Heat Exchange
Isobaric processes involve energy transfer primarily through heat exchange at constant pressure, allowing the system to perform work by expanding or compressing while maintaining pressure. In contrast, adiabatic processes occur without heat exchange, where energy transfer happens solely through work done on or by the system, resulting in temperature changes due to internal energy variations. These fundamental differences impact thermodynamic efficiency, with adiabatic processes often modeling rapid or insulated system changes and isobaric processes reflecting constant-pressure heat transfer scenarios.
Pressure, Volume, and Temperature Relationships
In an isobaric process, pressure remains constant while volume and temperature change proportionally according to Charles's Law, meaning volume increases linearly with temperature. In contrast, an adiabatic process involves no heat exchange, causing both pressure and temperature to change with volume according to the adiabatic condition \( P V^\gamma = \text{constant} \), where \( \gamma \) is the heat capacity ratio. Pressure decreases during expansion in an adiabatic process more significantly than in an isobaric process, as volume changes are coupled with temperature shifts without heat transfer.
Real-Life Examples of Isobaric and Adiabatic Processes
In an isobaric process, pressure remains constant while the volume changes, commonly observed in boiling water where heat causes water to vaporize at constant atmospheric pressure. Adiabatic processes occur without heat exchange, exemplified by the rapid compression of air in a bicycle pump, which increases temperature due to work done on the gas. Both processes are fundamental in thermodynamics, with isobaric processes prevalent in everyday heating systems and adiabatic processes essential in atmospheric phenomena like cloud formation and weather dynamics.
Applications in Engineering and Physics
Isobaric processes, characterized by constant pressure, are crucial in engineering applications such as internal combustion engines and refrigeration cycles where heat transfer at steady pressure improves system efficiency. Adiabatic processes, involving no heat exchange, are fundamental in thermodynamics for modeling rapid gas expansions or compressions in turbines, compressors, and atmospheric studies, ensuring energy conservation in isolated systems. Both processes underpin the design of heat engines, HVAC systems, and aero-engine performance analyses by optimizing energy transfer and thermodynamic cycle efficiency.
Summary and Comparative Analysis
Isobaric processes maintain constant pressure while allowing volume and temperature to change, governed by the relation \( P = \text{constant} \), and typically involve heat exchange with the surroundings. Adiabatic processes occur without heat transfer, causing internal energy changes to directly affect pressure and volume, described by \( PV^\gamma = \text{constant} \) where \( \gamma \) is the heat capacity ratio. Comparing both, isobaric processes involve heat flow and fixed pressure, whereas adiabatic processes preserve energy internally with no heat exchange and variable pressure, making them fundamental in thermodynamic cycle analyses such as Otto and Diesel engines.
Isobaric process Infographic
 libterm.com
libterm.com