Galvanization is a process that involves coating steel or iron with a layer of zinc to protect against corrosion and extend the lifespan of metal structures. This protective zinc layer acts as a barrier and provides sacrificial protection, preventing rust even if the coating is scratched. Discover how galvanization can enhance your metal's durability and the various methods used in this essential industrial treatment.
Table of Comparison
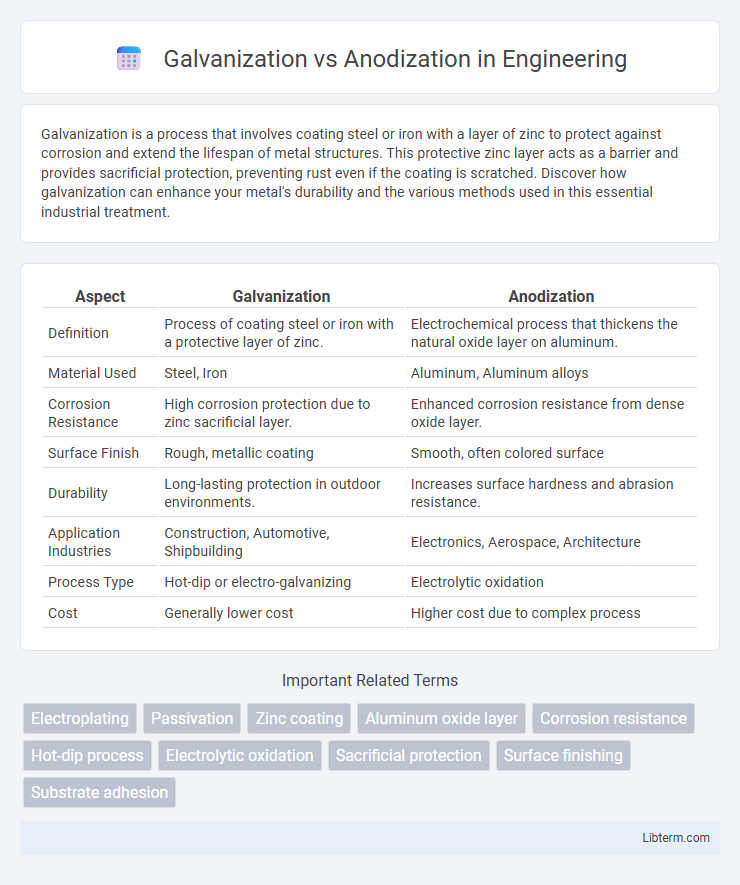
| Aspect | Galvanization | Anodization |
|---|---|---|
| Definition | Process of coating steel or iron with a protective layer of zinc. | Electrochemical process that thickens the natural oxide layer on aluminum. |
| Material Used | Steel, Iron | Aluminum, Aluminum alloys |
| Corrosion Resistance | High corrosion protection due to zinc sacrificial layer. | Enhanced corrosion resistance from dense oxide layer. |
| Surface Finish | Rough, metallic coating | Smooth, often colored surface |
| Durability | Long-lasting protection in outdoor environments. | Increases surface hardness and abrasion resistance. |
| Application Industries | Construction, Automotive, Shipbuilding | Electronics, Aerospace, Architecture |
| Process Type | Hot-dip or electro-galvanizing | Electrolytic oxidation |
| Cost | Generally lower cost | Higher cost due to complex process |
Introduction to Surface Treatment Processes
Galvanization involves coating steel or iron with a layer of zinc to prevent corrosion through a sacrificial barrier, enhancing durability especially in outdoor and industrial environments. Anodization is an electrochemical process primarily applied to aluminum, creating a thick oxide layer that improves corrosion resistance, surface hardness, and provides a base for dyeing. Both surface treatment processes extend the lifespan of metals but differ in application methods, materials treated, and protective mechanisms.
Understanding Galvanization
Galvanization is a corrosion protection process that applies a zinc coating to steel or iron, preventing rust by acting as a sacrificial anode. This electrochemical barrier effectively extends the lifespan of metal structures, especially in outdoor or harsh environments. The most common method, hot-dip galvanizing, involves immersing metal parts in molten zinc to ensure a durable and uniform protective layer.
Exploring Anodization
Anodization enhances aluminum surfaces by creating a durable oxide layer that improves corrosion resistance, wear resistance, and aesthetic appeal. This electrochemical process increases surface thickness, enabling superior paint adhesion and color customization without compromising the base metal's properties. Unlike galvanization, which coats steel with zinc, anodization specifically targets aluminum and alloys, offering lightweight protection tailored for architectural and aerospace applications.
Galvanization vs Anodization: Key Differences
Galvanization involves coating steel or iron with a protective layer of zinc to prevent rusting, while anodization enhances the natural oxide layer on aluminum to improve corrosion resistance and surface durability. The galvanization process is primarily used for ferrous metals, offering robust protection against corrosion, whereas anodization is specific to aluminum alloys, providing a decorative finish and increased hardness. Differences in coating thickness, corrosion resistance, and application methods define the key distinctions between galvanization and anodization in metal treatment.
Materials Suitable for Galvanization and Anodization
Galvanization is primarily suitable for ferrous metals like steel and iron, providing a protective zinc coating that prevents rust and corrosion. Anodization applies mainly to aluminum and its alloys, enhancing surface durability, corrosion resistance, and aesthetic appeal by thickening the natural oxide layer. Both processes cater to different base materials, with galvanization optimized for steel substrates and anodization specialized for aluminum components.
Protective Qualities and Corrosion Resistance
Galvanization provides superior corrosion resistance by coating metals, typically steel or iron, with a protective layer of zinc that prevents rust and extends the lifespan of outdoor structures. Anodization enhances aluminum surfaces by thickening the natural oxide layer, improving resistance to corrosion and wear while maintaining the metal's aesthetic appeal. Both methods offer robust protection, but galvanization is more effective for ferrous metals, whereas anodization is tailored for aluminum alloys with increased durability and corrosion resistance.
Aesthetic and Finish Considerations
Galvanization provides a robust, matte gray finish that resists corrosion but offers limited color options, ideal for industrial aesthetics. Anodization enhances aluminum surfaces with vibrant, long-lasting colors and a glossy or satin finish, allowing for greater customization and decorative appeal. Both processes improve durability, but anodization excels in producing visually striking, anodic layers that retain brightness and texture over time.
Cost and Application Environments
Galvanization offers a cost-effective corrosion resistance method ideal for outdoor steel structures exposed to harsh weather and industrial environments, providing durable zinc coating at lower initial expenses. Anodization, while generally more expensive due to its electrochemical process, enhances aluminum's surface hardness and corrosion resistance, making it suitable for aerospace, automotive, and decorative applications where aesthetic appeal and lightweight properties are critical. Choosing between galvanization and anodization depends on budget constraints and specific environmental factors such as exposure to chemicals, moisture, and mechanical wear.
Environmental Impact of Both Processes
Galvanization involves coating metal with zinc to prevent corrosion, but it produces hazardous waste and emits greenhouse gases during zinc extraction and electroplating. Anodization enhances aluminum surfaces by forming a protective oxide layer using electrolytic oxidation, generating less toxic waste and consuming lower energy levels, making it more environmentally friendly. Comparing both, anodization offers a reduced environmental footprint due to its cleaner process and sustainable material usage.
Choosing the Right Surface Treatment for Your Project
Galvanization provides a durable zinc coating that protects steel and iron from corrosion, making it ideal for outdoor and industrial applications requiring long-lasting rust resistance. Anodization enhances aluminum surfaces by increasing corrosion resistance and wear resistance while allowing for decorative color finishes, suited for aesthetic and lightweight components. Selecting the right surface treatment depends on the base metal, environmental exposure, desired appearance, and mechanical performance requirements of the project.
Galvanization Infographic
 libterm.com
libterm.com