A polytropic process describes the thermodynamic behavior of a gas undergoing a transformation where pressure and volume follow the relation \(PV^n = \text{constant}\), with \(n\) being the polytropic index. This process generalizes common processes such as isothermal, adiabatic, and isobaric by varying the value of \(n\). Discover how understanding the polytropic process can optimize your thermodynamic system's performance by reading further.
Table of Comparison
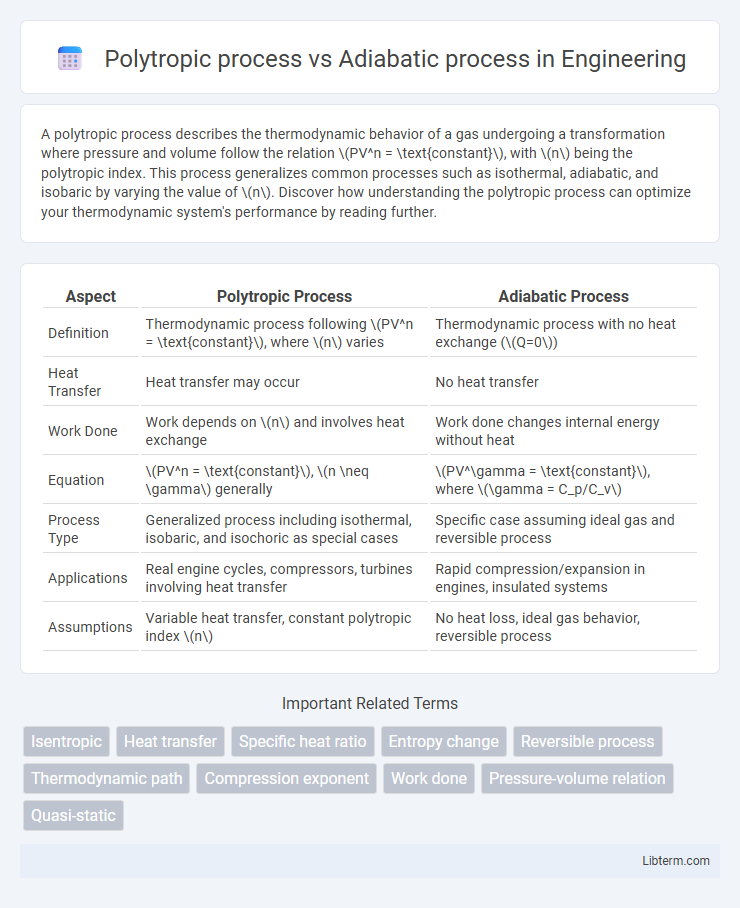
| Aspect | Polytropic Process | Adiabatic Process |
|---|---|---|
| Definition | Thermodynamic process following \(PV^n = \text{constant}\), where \(n\) varies | Thermodynamic process with no heat exchange (\(Q=0\)) |
| Heat Transfer | Heat transfer may occur | No heat transfer |
| Work Done | Work depends on \(n\) and involves heat exchange | Work done changes internal energy without heat |
| Equation | \(PV^n = \text{constant}\), \(n \neq \gamma\) generally | \(PV^\gamma = \text{constant}\), where \(\gamma = C_p/C_v\) |
| Process Type | Generalized process including isothermal, isobaric, and isochoric as special cases | Specific case assuming ideal gas and reversible process |
| Applications | Real engine cycles, compressors, turbines involving heat transfer | Rapid compression/expansion in engines, insulated systems |
| Assumptions | Variable heat transfer, constant polytropic index \(n\) | No heat loss, ideal gas behavior, reversible process |
Introduction to Thermodynamic Processes
Polytropic processes describe thermodynamic changes where pressure and volume follow \( PV^n = \text{constant} \), with the polytropic index \( n \) varying based on heat transfer conditions. Adiabatic processes are a specific case of polytropic processes with \( n = \gamma \) (ratio of specific heats) and occur without heat exchange, meaning all energy transfer is work. Understanding these processes is crucial in thermodynamics for analyzing energy transformations in engines, compressors, and turbines.
Definition of Polytropic Process
A polytropic process is a thermodynamic process that follows the relation \( PV^n = \text{constant} \), where \( P \) is pressure, \( V \) is volume, and \( n \) is the polytropic index representing the specific heat transfer characteristics of the process. Unlike an adiabatic process, where no heat transfer occurs and \( n \) equals the specific heat ratio \( \gamma \), the polytropic process allows for heat exchange with the surroundings, making it more general and applicable to real-world scenarios. The value of \( n \) determines the nature of the process, ranging from isothermal (\( n=1 \)) to adiabatic (\( n=\gamma \)) conditions.
Definition of Adiabatic Process
An adiabatic process is a thermodynamic transformation in which no heat exchange occurs between the system and its surroundings, ensuring that all energy changes are due to work done on or by the system. This process is characterized by constant entropy for reversible adiabatic conditions, making it distinct from polytropic processes where heat transfer may occur. In contrast, polytropic processes follow a generalized equation \(PV^n = \text{constant}\), with \(n\) varying to represent different types of processes, including isothermal and adiabatic, where the adiabatic case specifically corresponds to \(n = \gamma\), the heat capacity ratio.
Key Differences Between Polytropic and Adiabatic Processes
Polytropic processes involve heat transfer with a variable specific heat ratio (n), while adiabatic processes occur without heat exchange, characterized by a specific heat ratio (g). In polytropic processes, pressure and volume follow the relation \(PV^n = \text{constant}\), whereas adiabatic processes adhere to \(PV^\gamma = \text{constant}\). The key difference lies in the heat interaction: polytropic processes can be either heat-absorbing or releasing, while adiabatic processes are strictly thermally insulated.
Mathematical Expressions and Equations
The polytropic process follows the equation \( PV^n = \text{constant} \), where \( n \) is the polytropic index representing heat exchange characteristics, allowing it to describe a range of thermodynamic processes including isothermal (\( n=1 \)) and adiabatic (\( n=\gamma \)) processes. The adiabatic process is a specific case of the polytropic process where no heat transfer occurs, mathematically expressed as \( PV^\gamma = \text{constant} \), with \( \gamma \) being the ratio of specific heats \( C_p/C_v \). Both processes use the ideal gas law \( PV = nRT \) to relate pressure, volume, and temperature, but differ in the value of \( n \) and the heat interaction assumptions.
Physical Interpretation and Real-World Examples
A polytropic process describes a thermodynamic transformation where pressure and volume change following the relation \( PV^n = \text{constant} \), encompassing heat exchange with the surroundings and including adiabatic processes as a special case when the polytropic index \( n \) equals the specific heat ratio \( \gamma \). An adiabatic process involves no heat transfer, meaning all internal energy changes result solely from work done on or by the system, commonly observed in rapid gas compression or expansion such as in diesel engine cylinders. Real-world applications of polytropic processes are found in compressors and turbines where heat transfer cannot be neglected, whereas adiabatic processes are idealized models important for understanding insulated system behavior and fast thermodynamic cycles.
Energy Transfer and Heat Exchange Analysis
In a polytropic process, the energy transfer involves both work done by the system and heat exchange with the surroundings, characterized by a specific polytropic index that governs the relationship between pressure and volume. The adiabatic process features no heat exchange, meaning all energy transfer occurs solely as work, maintaining constant entropy throughout the process. Understanding these differences is critical for accurate thermodynamic modeling in engines and compressors where heat transfer and work interactions significantly impact performance.
Pressure-Volume Relationship Comparison
The pressure-volume relationship in a polytropic process follows the equation \( P V^n = \text{constant} \), where \( n \) is the polytropic index that varies between processes, capturing heat transfer effects. In contrast, an adiabatic process is a specific case of the polytropic process with no heat exchange, where \( n = \gamma \), the heat capacity ratio, resulting in the relation \( P V^\gamma = \text{constant} \). This difference causes the pressure in an adiabatic process to change more rapidly with volume compared to polytropic processes where \( n \ne \gamma \).
Applications in Engineering and Industry
Polytropic and adiabatic processes are critical in thermodynamic applications, especially in compressor and turbine design, where polytropic processes more accurately model real-world heat and work interactions. Polytropic processes are preferred in engineering calculations involving gas compression and expansion with heat transfer effects, optimizing efficiency in HVAC systems and internal combustion engines. Adiabatic processes, representing ideal insulation conditions, are essential for analyzing rapid, insulated expansions or compressions in pistons and nozzles, guiding the design of efficient propulsion and power generation systems.
Summary and Conclusion
The polytropic process follows the relation \(PV^n = \text{constant}\), where the exponent \(n\) varies depending on heat transfer, including isothermal, isobaric, and adiabatic as special cases. The adiabatic process is a specific polytropic process with no heat exchange, characterized by \(PV^\gamma = \text{constant}\), where \(\gamma\) is the heat capacity ratio \(C_p/C_v\). Understanding these distinctions is crucial in thermodynamics for accurately analyzing work and heat transfer in various engineering systems.
Polytropic process Infographic
 libterm.com
libterm.com