Diffusion is the process by which molecules move from an area of higher concentration to one of lower concentration, facilitating the equilibration of substances across membranes or within fluids. This natural phenomenon is essential for respiratory gas exchange, nutrient absorption, and waste removal in biological systems. Discover how diffusion shapes vital processes and why understanding it can enhance your grasp of cellular function in the detailed article ahead.
Table of Comparison
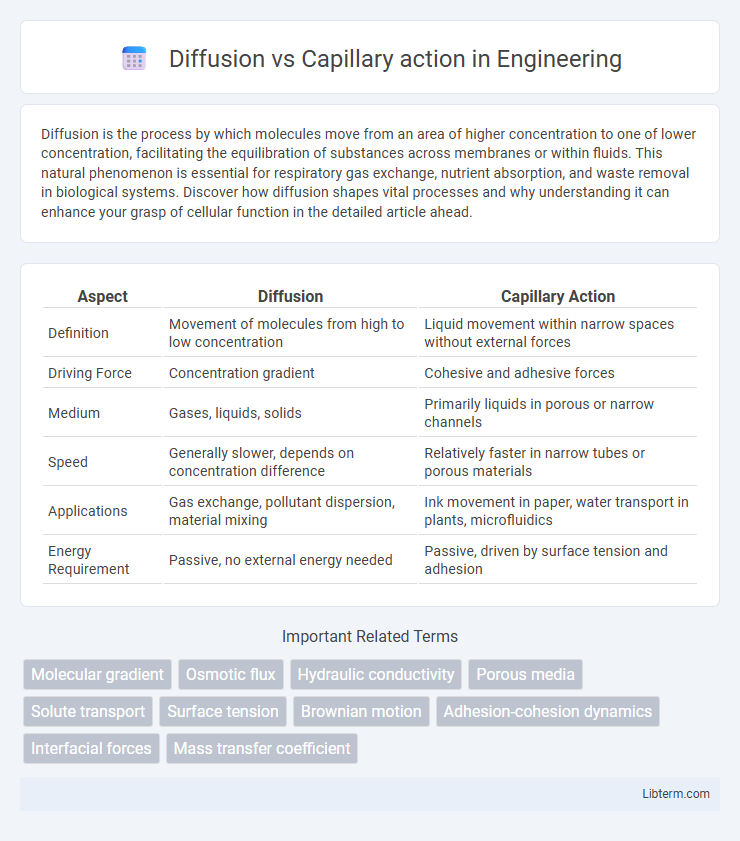
| Aspect | Diffusion | Capillary Action |
|---|---|---|
| Definition | Movement of molecules from high to low concentration | Liquid movement within narrow spaces without external forces |
| Driving Force | Concentration gradient | Cohesive and adhesive forces |
| Medium | Gases, liquids, solids | Primarily liquids in porous or narrow channels |
| Speed | Generally slower, depends on concentration difference | Relatively faster in narrow tubes or porous materials |
| Applications | Gas exchange, pollutant dispersion, material mixing | Ink movement in paper, water transport in plants, microfluidics |
| Energy Requirement | Passive, no external energy needed | Passive, driven by surface tension and adhesion |
Introduction to Diffusion and Capillary Action
Diffusion is the process where molecules move from an area of higher concentration to an area of lower concentration, driven by the concentration gradient until equilibrium is reached. Capillary action refers to the ability of a liquid to flow in narrow spaces without the assistance of external forces, largely influenced by adhesion, cohesion, and surface tension. Both diffusion and capillary action play critical roles in biological systems, fluid transport, and chemical processes.
Defining Diffusion: Key Concepts
Diffusion is the process where molecules move from an area of higher concentration to one of lower concentration, driven by the concentration gradient and thermal motion. It occurs in gases, liquids, and solids, enabling the passive transport of substances without energy input. Key concepts include random molecular movement, equilibrium attainment, and dependence on factors such as temperature, concentration difference, and medium permeability.
Understanding Capillary Action: Basic Principles
Capillary action occurs when liquid spontaneously rises or moves through narrow spaces due to adhesion, cohesion, and surface tension forces. This phenomenon is critical in processes such as water transport in plants, where adhesion between water molecules and plant cell walls overcomes gravity. Unlike diffusion, which involves the movement of molecules from high to low concentration, capillary action relies on intermolecular forces and tube diameter to drive fluid movement.
Mechanisms Driving Diffusion
Diffusion is driven by the random thermal motion of molecules moving from an area of higher concentration to lower concentration, resulting in a net movement that equalizes solute distribution. This process relies on concentration gradients without requiring external forces or structures. In contrast, capillary action depends on adhesive and cohesive forces that allow liquid to move through narrow spaces against gravity, primarily influenced by surface tension.
How Capillary Action Works
Capillary action occurs when liquid spontaneously rises or falls within narrow spaces due to the adhesive force between the liquid and the solid surface being stronger than the cohesive forces within the liquid. The interplay of surface tension and adhesion causes the liquid to climb against gravity in thin tubes or porous materials. This phenomenon is crucial in processes like water transport in plants and ink movement in paper, contrasting with diffusion, which relies on molecular concentration gradients without directional force.
Factors Influencing Diffusion
Diffusion is influenced by factors such as concentration gradient, temperature, molecule size, and medium density, which determine the rate at which particles move from high to low concentration areas. Higher temperatures increase kinetic energy and diffusion speed, while larger molecules diffuse more slowly. The diffusion process differs from capillary action, which depends on surface tension, adhesion, and cohesion to move liquids through narrow spaces like plant xylem or porous materials.
Determinants Affecting Capillary Action
Capillary action is primarily affected by the adhesive forces between the liquid and the solid surface, as well as the cohesive forces within the liquid itself. The diameter of the capillary tube, the surface tension of the liquid, and the contact angle between the liquid and the surface are critical determinants influencing the height and rate of capillary rise. Unlike diffusion, which depends largely on concentration gradients and molecular motion, capillary action is driven by intermolecular forces and the physical properties of the liquid-solid interface.
Real-World Applications of Diffusion
Diffusion drives essential processes such as oxygen transport from alveoli to blood in the lungs and nutrient absorption in the intestines, enabling cellular respiration and metabolism. It plays a critical role in industrial applications like gas separation, wastewater treatment, and drug delivery systems where molecules move from high to low concentration effortlessly. Unlike capillary action, which relies on adhesion forces for fluid movement in narrow spaces, diffusion depends on molecular concentration gradients for mass transfer across membranes and porous materials.
Practical Uses of Capillary Action
Capillary action enables water movement in soil, facilitating plant hydration and nutrient absorption vital for agriculture and gardening. It supports ink flow in pens and the rise of liquids in thin tubes, critical for medical devices like blood samplers and diagnostic test strips. This phenomenon also aids in textile drying and oil absorption in porous materials, making it essential for manufacturing and environmental cleanup.
Diffusion vs Capillary Action: Key Differences and Comparison
Diffusion is the passive movement of molecules from an area of higher concentration to lower concentration due to random molecular motion, while capillary action involves the movement of liquid within narrow spaces without external forces, driven by adhesive and cohesive forces. Diffusion occurs in gases, liquids, and solids and is governed by concentration gradients, whereas capillary action primarily occurs in liquids interacting with solid surfaces, influenced by surface tension and adhesion. Key differences include the driving forces--diffusion relies on concentration gradients, capillary action on intermolecular forces--and the mediums, with diffusion involving molecular mixing and capillary action involving liquid rise or movement in narrow tubes or porous materials.
Diffusion Infographic
 libterm.com
libterm.com