Brittleness refers to a material's tendency to break or shatter without significant deformation when subjected to stress. This property is critical in engineering and manufacturing, where the choice of materials must balance strength and flexibility to prevent sudden failure. Explore this article to understand how brittleness impacts material selection and product durability in your projects.
Table of Comparison
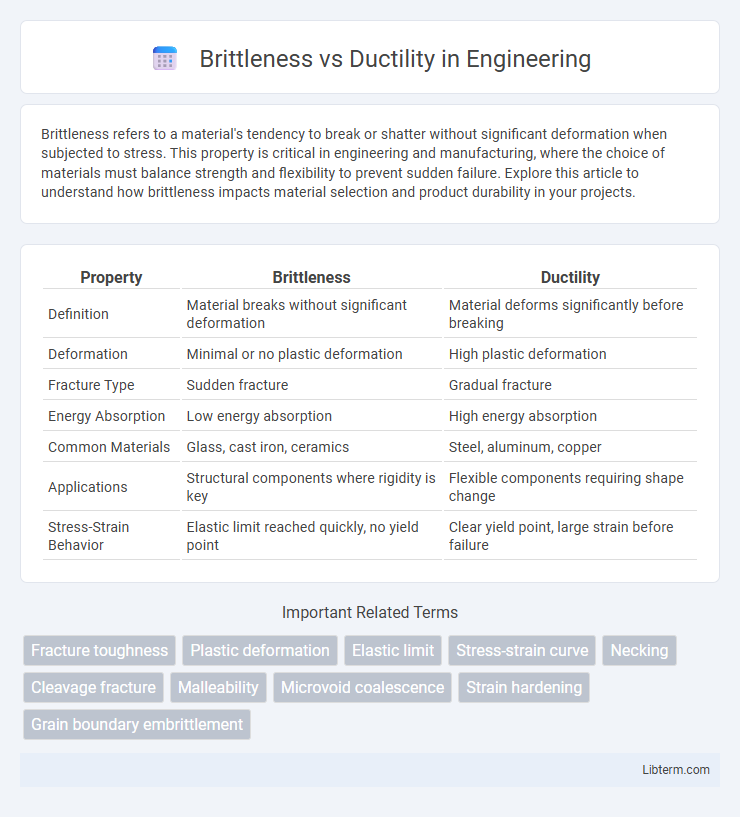
| Property | Brittleness | Ductility |
|---|---|---|
| Definition | Material breaks without significant deformation | Material deforms significantly before breaking |
| Deformation | Minimal or no plastic deformation | High plastic deformation |
| Fracture Type | Sudden fracture | Gradual fracture |
| Energy Absorption | Low energy absorption | High energy absorption |
| Common Materials | Glass, cast iron, ceramics | Steel, aluminum, copper |
| Applications | Structural components where rigidity is key | Flexible components requiring shape change |
| Stress-Strain Behavior | Elastic limit reached quickly, no yield point | Clear yield point, large strain before failure |
Introduction to Brittleness and Ductility
Brittleness describes a material's tendency to fracture without significant deformation when subjected to stress, often seen in ceramics and glass. Ductility refers to a material's ability to undergo plastic deformation before rupture, commonly observed in metals like copper and aluminum. Understanding the contrast between brittleness and ductility is essential for selecting materials in engineering applications where mechanical performance under load is critical.
Defining Brittleness: Key Characteristics
Brittleness is defined by a material's inability to undergo significant plastic deformation before fracture, causing it to break suddenly under stress. Key characteristics include low toughness, high hardness, and a tendency to crack rather than bend when subjected to impact or tensile forces. Examples of brittle materials are ceramics, glass, and cast iron, which fracture without noticeable deformation.
Understanding Ductility: Essential Properties
Ductility refers to a material's ability to undergo significant plastic deformation before rupture, characterized by high tensile strength and elongation capacity. It is essential in metals like copper and aluminum, allowing them to be drawn into wires or stretched without breaking. Understanding ductility helps engineers select materials that absorb energy and deform safely under stress, contrasting with brittle materials that fracture with minimal deformation.
Atomic Structure and Its Influence
Brittleness and ductility in materials are fundamentally influenced by atomic structure, particularly the arrangement and bonding of atoms. In brittle materials, strong directional covalent or ionic bonds restrict dislocation movement, leading to fracture without significant plastic deformation, while ductile materials possess metallic bonds with non-directional electron clouds allowing atoms to slide past each other easily. The presence of slip systems and the ability of dislocations to move within the crystal lattice directly correlate with enhanced ductility, whereas limited slip systems and rigid lattice structures increase brittleness.
Mechanical Behavior Under Stress
Brittleness refers to a material's tendency to fracture without significant deformation when subjected to stress, often breaking suddenly under tensile or impact loads. Ductility characterizes a material's ability to undergo substantial plastic deformation before failure, allowing it to absorb energy and stretch under tensile stress. Understanding the stress-strain relationship is crucial for predicting mechanical behavior, with brittle materials showing a steep elastic region and minimal plastic deformation, while ductile materials exhibit extensive plastic deformation before fracturing.
Factors Affecting Brittleness and Ductility
Temperature significantly influences brittleness and ductility, as materials generally become more brittle at lower temperatures and more ductile at higher temperatures. The crystal structure and grain size of metals impact these properties, with finer grains typically enhancing ductility while coarse grains may increase brittleness. Alloying elements and the presence of impurities also alter the balance between brittleness and ductility by affecting atomic bonding and dislocation movement within the material.
Material Examples: Brittle vs. Ductile
Glass and ceramics are prime examples of brittle materials, as they break with little deformation under stress due to their atomic structure. In contrast, metals like copper and aluminum exhibit ductility, characterized by their ability to undergo significant plastic deformation before fracture. Understanding the distinction between brittle and ductile behavior is crucial for selecting materials in engineering applications requiring either rigidity or flexibility.
Industrial and Engineering Applications
Brittleness in industrial materials often leads to sudden failure without significant deformation, posing risks in critical engineering applications like construction and aerospace where impact resistance is essential. Ductility allows materials such as steel and aluminum alloys to undergo plastic deformation, enabling energy absorption and safer failure modes in structural components, automotive manufacturing, and pipeline systems. Understanding the balance between brittleness and ductility is crucial for selecting appropriate materials to ensure durability, safety, and performance in engineering designs.
Failure Modes: Fracture vs. Deformation
Brittleness is characterized by materials failing through fracture with little to no plastic deformation, often resulting in sudden and catastrophic breaks under stress. Ductile materials typically undergo significant plastic deformation before failure, allowing them to absorb energy and deform noticeably rather than fracturing abruptly. Understanding the failure modes--fracture in brittle materials versus deformation in ductile ones--is crucial for selecting materials in applications requiring resilience or energy absorption.
Selecting Materials: Practical Considerations
Selecting materials based on brittleness versus ductility involves evaluating mechanical properties such as tensile strength, fracture toughness, and elongation at break to ensure performance under expected loads and environmental conditions. Brittle materials like ceramics offer high compressive strength but low deformation before failure, making them ideal for applications requiring rigidity but susceptible to crack propagation. Ductile materials such as metals provide significant plastic deformation, allowing energy absorption and resistance to impact, which is crucial for safety-critical components in automotive and aerospace industries.
Brittleness Infographic
 libterm.com
libterm.com