Electrochemical energy harnesses chemical reactions to generate electrical power, playing a critical role in batteries and fuel cells. This form of energy conversion is essential for portable electronics, electric vehicles, and renewable energy storage solutions. Explore the rest of the article to understand how electrochemical energy can power your future innovations.
Table of Comparison
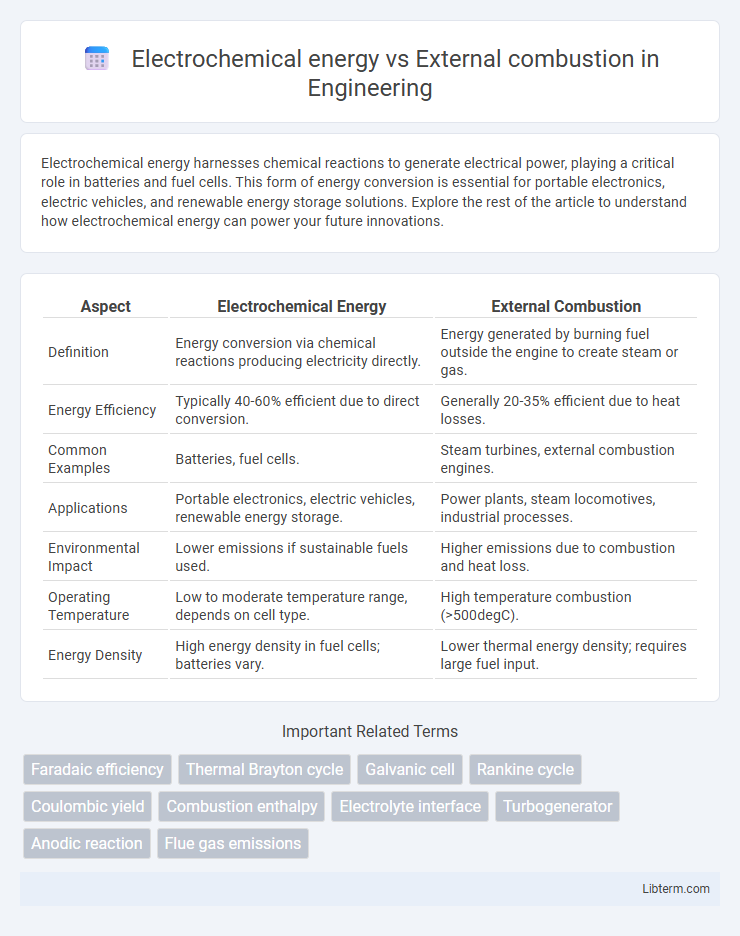
| Aspect | Electrochemical Energy | External Combustion |
|---|---|---|
| Definition | Energy conversion via chemical reactions producing electricity directly. | Energy generated by burning fuel outside the engine to create steam or gas. |
| Energy Efficiency | Typically 40-60% efficient due to direct conversion. | Generally 20-35% efficient due to heat losses. |
| Common Examples | Batteries, fuel cells. | Steam turbines, external combustion engines. |
| Applications | Portable electronics, electric vehicles, renewable energy storage. | Power plants, steam locomotives, industrial processes. |
| Environmental Impact | Lower emissions if sustainable fuels used. | Higher emissions due to combustion and heat loss. |
| Operating Temperature | Low to moderate temperature range, depends on cell type. | High temperature combustion (>500degC). |
| Energy Density | High energy density in fuel cells; batteries vary. | Lower thermal energy density; requires large fuel input. |
Introduction to Electrochemical Energy and External Combustion
Electrochemical energy involves the direct conversion of chemical energy into electrical energy through redox reactions, commonly seen in batteries and fuel cells where ions move between electrodes. External combustion engines convert chemical energy into mechanical work by burning fuel outside the engine, producing heat that drives a working fluid like steam or gas. Electrochemical systems offer higher efficiency and cleaner energy conversion compared to traditional external combustion processes, which rely on thermodynamic cycles such as the Rankine or Brayton cycles.
Fundamental Principles of Electrochemical Energy
Electrochemical energy conversion relies on redox reactions where chemical energy is directly transformed into electrical energy through electron transfer between electrodes and electrolyte. This process is governed by the fundamental principles of electrochemistry, including the Nernst equation, electrode potentials, and ionic conductivity. Unlike external combustion systems that rely on heat engines and thermodynamic cycles, electrochemical systems utilize electrochemical cells such as batteries and fuel cells to efficiently generate electricity at the molecular level.
Basic Mechanics of External Combustion Systems
External combustion systems operate by burning fuel outside the engine to generate heat, which then produces steam or hot gases to drive mechanical work. These systems rely on a boiler or furnace where combustion occurs, transferring energy through heat exchangers to power turbines or pistons. Unlike electrochemical energy systems that convert chemical energy directly into electricity via reactions in batteries or fuel cells, external combustion focuses on thermal energy conversion and mechanical motion.
Comparative Energy Conversion Efficiencies
Electrochemical energy systems, such as fuel cells and batteries, typically achieve energy conversion efficiencies between 40% and 60%, surpassing external combustion engines which often operate at 20% to 30% efficiency. Fuel cells convert chemical energy directly into electrical energy through redox reactions, minimizing energy losses associated with heat transfer in external combustion. External combustion engines, relying on thermal cycles like the Rankine or Brayton, face inherent inefficiencies from heat dissipation and mechanical friction, leading to lower overall efficiency compared to electrochemical methods.
Environmental Impact: Emissions and Byproducts
Electrochemical energy systems, such as fuel cells and batteries, produce minimal emissions, typically only water vapor or oxygen as byproducts, significantly reducing air pollution and greenhouse gases. In contrast, external combustion engines, like steam turbines burning fossil fuels, emit substantial quantities of CO2, NOx, and particulate matter, contributing to climate change and respiratory health issues. The clean nature of electrochemical energy sources makes them a more environmentally sustainable option compared to the high-emission profile of external combustion systems.
Applications in Modern Technology
Electrochemical energy systems, such as batteries and fuel cells, dominate modern portable electronics, electric vehicles, and renewable energy storage due to their high efficiency and compact design. External combustion engines, found in traditional power plants and steam turbines, are primarily used for large-scale electricity generation and industrial processes requiring continuous, controlled heat output. The shift toward sustainable technology increasingly favors electrochemical energy for its lower emissions and adaptability in decentralized energy applications.
Scalability and Infrastructure Requirements
Electrochemical energy systems, such as batteries and fuel cells, offer high scalability with modular designs that can easily expand from small-scale applications to large grid storage solutions. These systems require specialized infrastructure like charging stations and maintenance facilities but benefit from faster deployment and lower spatial footprints compared to external combustion power plants. External combustion systems, including coal and natural gas plants, demand extensive infrastructure investments, significant land use, and complex fuel supply chains, limiting their scalability and flexibility in rapidly changing energy markets.
Cost Analysis: Investment and Maintenance
Electrochemical energy systems, such as batteries and fuel cells, typically involve higher upfront investment costs due to advanced materials and complex manufacturing processes but benefit from lower maintenance expenses and longer operational lifespans. External combustion engines, relying on steam generation from external fuel sources, usually require lower initial capital investment but incur higher ongoing maintenance costs stemming from mechanical wear, fuel handling, and emissions control. Cost analysis must consider the total cost of ownership, factoring in initial investment, efficiency, operational expenses, and maintenance demands, where electrochemical systems often show economic advantages in long-term applications.
Future Prospects and Innovations
Electrochemical energy systems are advancing rapidly with innovations in solid-state batteries and hydrogen fuel cells promising higher efficiency and longer lifespans compared to traditional external combustion engines. Ongoing research into nanomaterials and catalysts enhances energy density and reduces costs, positioning electrochemical technologies as a cornerstone for sustainable transport and grid storage. While external combustion engines continue to evolve with cleaner fuels and hybrid designs, their future prospects are overshadowed by the scalability and environmental benefits of electrochemical alternatives.
Summary: Choosing the Right Energy Solution
Electrochemical energy systems, such as batteries and fuel cells, offer high efficiency and direct energy conversion with minimal emissions, making them ideal for portable and renewable energy applications. External combustion engines, including steam turbines, provide greater fuel flexibility and substantial power output but often suffer from lower efficiency and higher operational complexity. Selecting the right energy solution depends on factors like energy density, environmental impact, application scale, and fuel availability, where electrochemical systems excel in clean, compact use cases while external combustion suits large-scale power generation.
Electrochemical energy Infographic
 libterm.com
libterm.com