Corrosion is the natural process where metals deteriorate due to chemical reactions with their environment, often involving moisture and oxygen. This degradation can lead to structural damage, safety hazards, and increased maintenance costs in industries ranging from construction to transportation. Explore the rest of this article to understand how corrosion impacts your assets and discover effective prevention strategies.
Table of Comparison
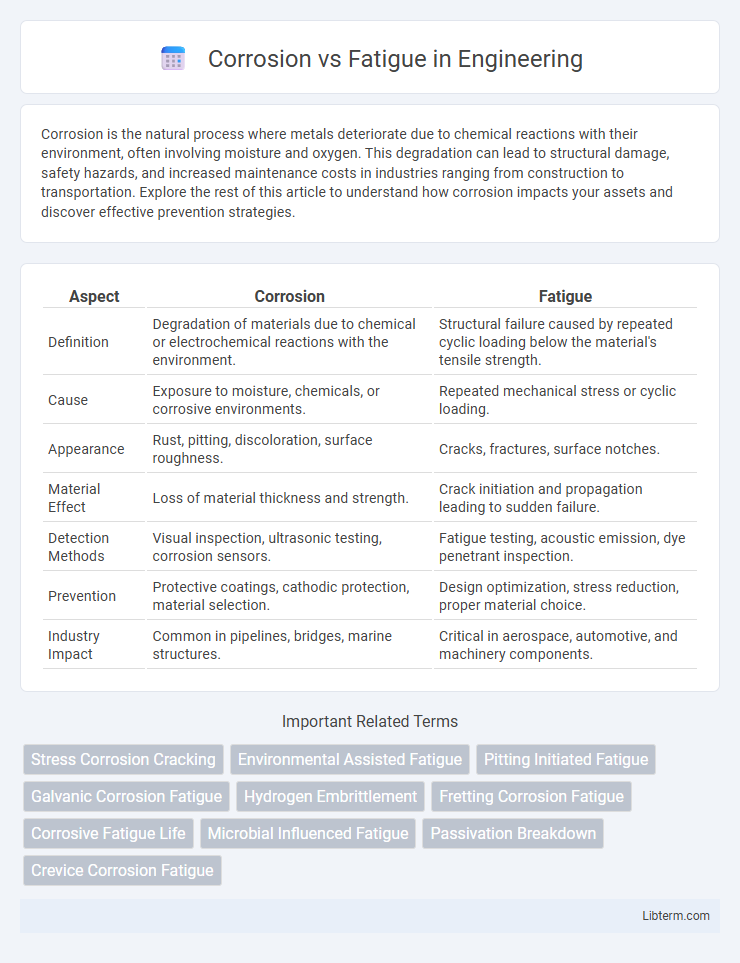
| Aspect | Corrosion | Fatigue |
|---|---|---|
| Definition | Degradation of materials due to chemical or electrochemical reactions with the environment. | Structural failure caused by repeated cyclic loading below the material's tensile strength. |
| Cause | Exposure to moisture, chemicals, or corrosive environments. | Repeated mechanical stress or cyclic loading. |
| Appearance | Rust, pitting, discoloration, surface roughness. | Cracks, fractures, surface notches. |
| Material Effect | Loss of material thickness and strength. | Crack initiation and propagation leading to sudden failure. |
| Detection Methods | Visual inspection, ultrasonic testing, corrosion sensors. | Fatigue testing, acoustic emission, dye penetrant inspection. |
| Prevention | Protective coatings, cathodic protection, material selection. | Design optimization, stress reduction, proper material choice. |
| Industry Impact | Common in pipelines, bridges, marine structures. | Critical in aerospace, automotive, and machinery components. |
Understanding Corrosion: Definition and Mechanisms
Corrosion is the chemical or electrochemical reaction between a material, primarily metals, and its environment, leading to the gradual deterioration of the material's properties. Common mechanisms include oxidation, where metal atoms lose electrons and form oxides, and galvanic corrosion, which occurs when two different metals are electrically connected in the presence of an electrolyte. Understanding the specific environmental factors, such as moisture, temperature, and pH, is crucial for predicting corrosion behavior and selecting appropriate protective measures.
Fatigue Explained: Causes and Process
Fatigue in materials occurs due to repeated cyclic loading causing the initiation and growth of microscopic cracks over time. This process begins with stress concentrations at surface defects or inclusions, leading to crack propagation under fluctuating stresses below the material's ultimate tensile strength. Understanding the fatigue process is critical for predicting the lifespan of components exposed to dynamic loads in industries such as aerospace, automotive, and structural engineering.
Key Differences Between Corrosion and Fatigue
Corrosion is the chemical or electrochemical reaction between a material, usually metal, and its environment leading to material degradation, whereas fatigue is the progressive and localized structural damage that occurs when a material is subjected to cyclic loading. Corrosion typically results in uniform material loss or pitting over time, while fatigue causes crack initiation and propagation due to repeated stress variations below the material's ultimate tensile strength. Corrosion influences the surface and reduces cross-sectional area, accelerating fatigue failure, but fatigue damage primarily originates from mechanical stress cycles independent of chemical reactions.
Common Materials Affected by Corrosion and Fatigue
Common materials affected by corrosion include steel, aluminum, and copper alloys, which often experience degradation in presence of moisture, salts, and acidic environments. Fatigue commonly impacts metals such as aluminum, titanium, and stainless steel, especially in components subjected to cyclic loading and mechanical stress. Both phenomena significantly reduce the lifespan and structural integrity of materials used in aerospace, automotive, and infrastructure applications.
Environmental Factors Influencing Corrosion
Environmental factors such as humidity, temperature, and the presence of corrosive agents like saltwater or industrial pollutants significantly accelerate corrosion processes in metals. Oxygen concentration, pH levels, and exposure to chemicals like acids or bases also intensify electrochemical reactions, leading to faster material degradation. These conditions undermine structural integrity, increasing the likelihood of corrosion-induced failures compared to fatigue damage influenced primarily by mechanical stress cycles.
Stress Factors Impacting Fatigue Life
Stress factors impacting fatigue life include cyclic loading amplitude, mean stress levels, and frequency of stress cycles, all of which accelerate material degradation under repeated stress. Corrosion introduces surface defects and micro-cracks that act as stress concentrators, reducing fatigue resistance and accelerating crack initiation. Environmental conditions such as humidity and presence of corrosive agents exacerbate these effects, significantly diminishing the overall fatigue life of metals.
Synergistic Effects: Corrosion Fatigue
Corrosion fatigue occurs when cyclic mechanical stress and corrosive environments combine, accelerating crack initiation and growth beyond the effects of fatigue or corrosion alone. The synergistic interaction reduces material life significantly in metals such as steel, aluminum alloys, and titanium, especially in marine and industrial applications. Understanding corrosion fatigue mechanisms is critical for designing durable components exposed to fluctuating stresses and aggressive chemical exposure.
Detection Methods for Corrosion vs Fatigue
Corrosion detection methods primarily involve visual inspections, ultrasonic testing, and corrosion coupons to identify material degradation and measure corrosion rates. Fatigue detection focuses on techniques such as acoustic emission monitoring, strain gauge analysis, and periodic non-destructive testing methods like dye penetrant and magnetic particle inspection to identify crack initiation and propagation. Advanced approaches combine sensor data and real-time monitoring systems to differentiate between corrosion-induced damage and fatigue-related cracks effectively.
Prevention and Mitigation Strategies
Corrosion prevention involves techniques such as protective coatings, cathodic protection, and corrosion inhibitors to extend material lifespan by minimizing chemical reactions with the environment. Fatigue mitigation focuses on design optimization, stress reduction, and regular inspections to detect early cracks and prevent material failure due to cyclic loading. Combining corrosion-resistant materials with fatigue-resistant design principles significantly enhances structural durability in harsh environments.
Real-World Examples: Corrosion and Fatigue Failures
Corrosion failures often occur in marine environments where saltwater accelerates metal degradation, exemplified by the collapse of the Silver Bridge in 1967 due to stress corrosion cracking. Fatigue failures are common in aerospace, with the 1988 Aloha Airlines incident caused by cyclic stresses leading to a catastrophic fuselage fracture. Both failure types highlight the critical need for material selection and maintenance strategies to prevent structural collapse in infrastructure and transportation industries.
Corrosion Infographic
 libterm.com
libterm.com