Anodizing enhances the natural oxide layer on metal surfaces, significantly increasing corrosion resistance and wear durability. This electrochemical process also allows for vibrant color finishes, improving both functionality and aesthetic appeal of aluminum and other metals. Explore the article to understand how anodizing can extend the life and beauty of your metal components.
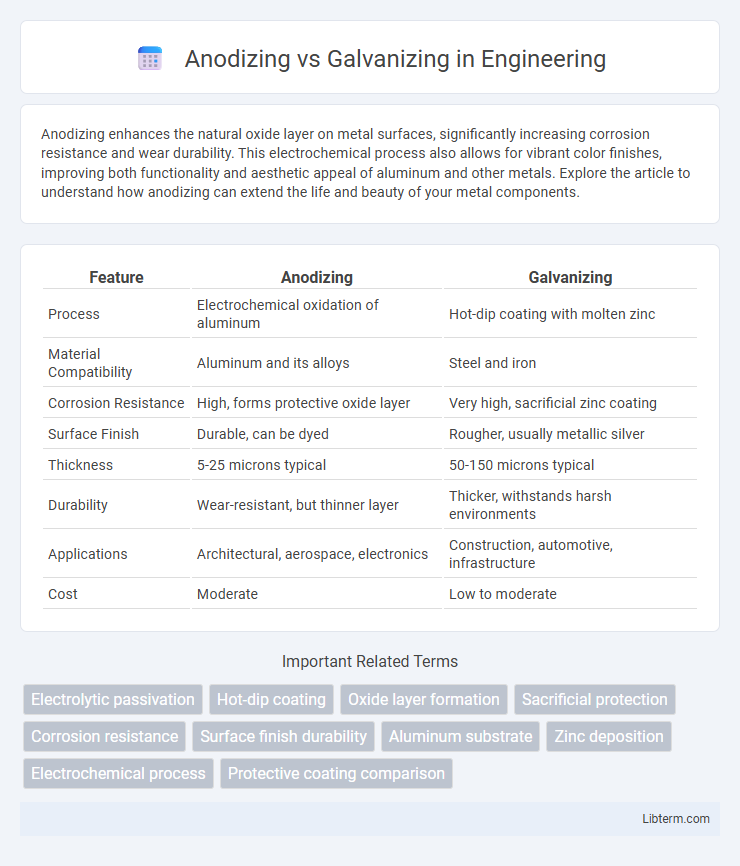
Table of Comparison
| Feature | Anodizing | Galvanizing |
|---|---|---|
| Process | Electrochemical oxidation of aluminum | Hot-dip coating with molten zinc |
| Material Compatibility | Aluminum and its alloys | Steel and iron |
| Corrosion Resistance | High, forms protective oxide layer | Very high, sacrificial zinc coating |
| Surface Finish | Durable, can be dyed | Rougher, usually metallic silver |
| Thickness | 5-25 microns typical | 50-150 microns typical |
| Durability | Wear-resistant, but thinner layer | Thicker, withstands harsh environments |
| Applications | Architectural, aerospace, electronics | Construction, automotive, infrastructure |
| Cost | Moderate | Low to moderate |
Introduction to Anodizing and Galvanizing
Anodizing is an electrochemical process that enhances the natural oxide layer on aluminum surfaces, improving corrosion resistance, durability, and aesthetic appeal. Galvanizing involves coating steel or iron with a layer of zinc through hot-dip or electroplating methods to protect against rust and corrosion. Both processes extend the lifespan of metals but differ in materials treated and protective mechanisms.
How Anodizing Works: Process Overview
Anodizing enhances aluminum's corrosion resistance and surface durability by creating a controlled oxide layer through an electrochemical process where the metal acts as an anode in an acid electrolyte. This oxide layer is porous initially, allowing dyes or sealants to be applied for color and additional protection before it fully hardens. Unlike galvanizing, which coats steel with zinc to prevent corrosion, anodizing permanently alters the aluminum surface for improved wear resistance and aesthetic appeal.
The Galvanizing Process Explained
The galvanizing process involves coating steel or iron with a protective layer of zinc to prevent corrosion and extend metal durability. This is typically achieved through hot-dip galvanizing, where the metal is submerged in molten zinc, forming a strong metallurgical bond that creates a corrosion-resistant barrier. Compared to anodizing, which enhances aluminum with an oxide layer, galvanizing offers superior protection for ferrous metals in harsh environmental conditions.
Key Differences Between Anodizing and Galvanizing
Anodizing enhances aluminum surfaces by creating a durable oxide layer through an electrochemical process, improving corrosion resistance and aesthetic appeal without adding significant thickness. Galvanizing involves coating steel or iron with a protective layer of zinc via hot-dip immersion or electroplating, providing robust corrosion protection primarily for ferrous metals. The key difference lies in anodizing being a surface treatment for non-ferrous metals like aluminum, enhancing color and finish options, while galvanizing physically applies a metal coating to ferrous metals, offering sacrificial protection against rust.
Material Compatibility: Metals Suitable for Each Process
Anodizing is primarily compatible with aluminum, titanium, and magnesium, enhancing corrosion resistance and surface hardness specifically on non-ferrous metals. Galvanizing, typically applied to steel and iron, involves zinc coating to prevent rust and provide durable protection in harsh environments. Understanding the distinct metal compatibilities ensures optimal corrosion protection and longevity for industrial and construction applications.
Corrosion Resistance: Anodizing vs Galvanizing
Anodizing enhances corrosion resistance by creating a thick oxide layer on aluminum surfaces, making it highly effective in protecting against oxidation and wear. Galvanizing involves coating steel or iron with a layer of zinc, offering sacrificial protection by corroding instead of the base metal, which is particularly beneficial in harsh environments. Both methods improve corrosion resistance but differ in metal compatibility and protective mechanisms, with anodizing suited for aluminum and galvanizing ideal for steel and iron applications.
Durability and Lifespan Comparison
Anodizing enhances aluminum durability by creating a thick oxide layer that resists corrosion, scratches, and wear, often extending the lifespan up to 20 years or more in outdoor environments. Galvanizing coats steel with a zinc layer that provides robust protection against rust and corrosion, typically lasting 30 to 50 years depending on environmental exposure. While anodizing offers superior aesthetic finish and moderate corrosion resistance primarily for aluminum, galvanizing delivers stronger long-term corrosion protection for steel structures, making it ideal for heavy-duty applications.
Aesthetic Options and Surface Finishes
Anodizing offers a wide range of vibrant color options and enhances surface hardness through an electrochemical process, creating a durable, corrosion-resistant oxide layer often favored for architectural and decorative applications. Galvanizing, typically resulting in a matte or spangled zinc coating, provides a rugged, protective finish primarily aimed at corrosion resistance rather than aesthetic customization. While anodizing allows for smooth, glossy, and color-consistent surfaces, galvanizing emphasizes functional surface protection with limited aesthetic versatility.
Common Applications in Industry
Anodizing is widely used in aerospace, automotive, and electronic industries to enhance aluminum's corrosion resistance and surface durability, making it ideal for aircraft components, automotive parts, and electronic enclosures. Galvanizing, primarily applied in construction, infrastructure, and agriculture, involves coating steel with zinc to protect structural steel beams, fencing, and outdoor machinery from rust and environmental damage. Both processes are critical in industries requiring enhanced metal longevity but differ in material compatibility and protective mechanisms.
Choosing the Right Protection Method for Your Project
Anodizing enhances aluminum's durability by creating a corrosion-resistant oxide layer that is integral to the material, ideal for lightweight applications requiring aesthetic finishes and electrical insulation. Galvanizing involves coating steel or iron with a layer of zinc, providing robust protection against rust and suitable for heavy-duty, outdoor structural projects exposed to harsh environments. Selecting between anodizing and galvanizing depends on the base metal, environmental exposure, and desired longevity, with anodizing favored for aluminum components and galvanizing preferred for steel frameworks.
Anodizing Infographic
 libterm.com
libterm.com