Vapour pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature. It plays a crucial role in processes such as evaporation, boiling, and condensation, influencing everything from weather patterns to industrial applications. Discover how understanding vapour pressure can impact your approach to these phenomena by reading the full article.
Table of Comparison
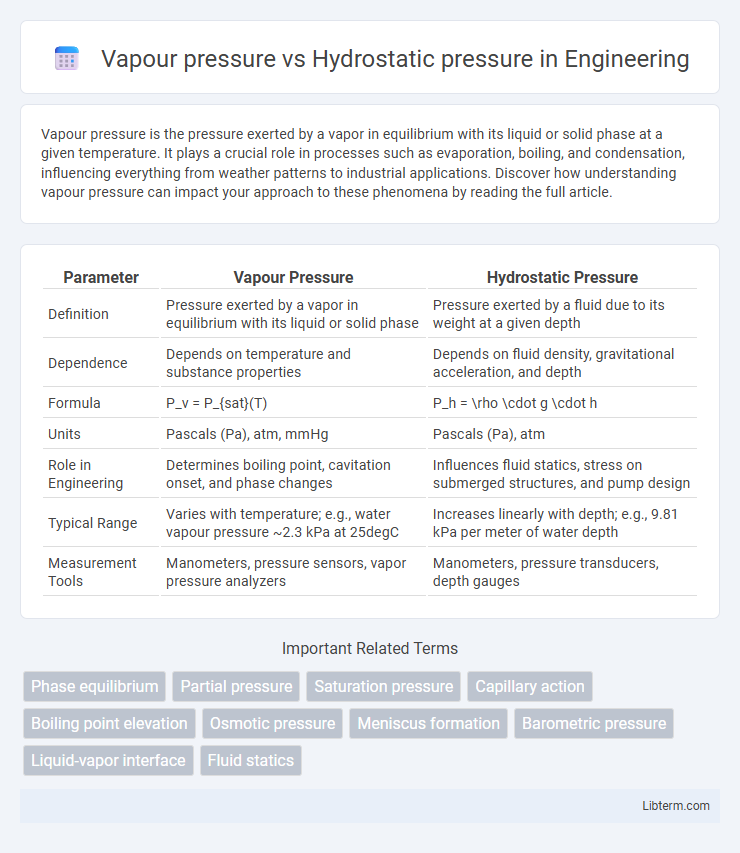
| Parameter | Vapour Pressure | Hydrostatic Pressure |
|---|---|---|
| Definition | Pressure exerted by a vapor in equilibrium with its liquid or solid phase | Pressure exerted by a fluid due to its weight at a given depth |
| Dependence | Depends on temperature and substance properties | Depends on fluid density, gravitational acceleration, and depth |
| Formula | P_v = P_{sat}(T) | P_h = \rho \cdot g \cdot h |
| Units | Pascals (Pa), atm, mmHg | Pascals (Pa), atm |
| Role in Engineering | Determines boiling point, cavitation onset, and phase changes | Influences fluid statics, stress on submerged structures, and pump design |
| Typical Range | Varies with temperature; e.g., water vapour pressure ~2.3 kPa at 25degC | Increases linearly with depth; e.g., 9.81 kPa per meter of water depth |
| Measurement Tools | Manometers, pressure sensors, vapor pressure analyzers | Manometers, pressure transducers, depth gauges |
Introduction to Vapour Pressure and Hydrostatic Pressure
Vapour pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its liquid or solid phase at a given temperature, reflecting a substance's tendency to evaporate. Hydrostatic pressure is the pressure exerted by a fluid at rest due to the force of gravity, increasing linearly with fluid depth and density. Understanding the differences between vapour pressure and hydrostatic pressure is crucial in fields such as fluid mechanics, meteorology, and chemical engineering.
Defining Vapour Pressure: Key Concepts
Vapour pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its liquid or solid phase at a given temperature, reflecting a substance's tendency to evaporate. It depends on temperature and the nature of the liquid, with higher vapor pressures indicating greater volatility. Hydrostatic pressure, by contrast, represents the pressure exerted by a fluid at equilibrium due to the force of gravity, increasing with depth and fluid density.
Understanding Hydrostatic Pressure: Core Principles
Hydrostatic pressure is the pressure exerted by a fluid at rest due to the force of gravity acting on its mass, calculated as the product of fluid density, gravitational acceleration, and fluid depth (P = rgh). It increases linearly with depth and is independent of the container shape, making it a fundamental concept in fluid mechanics, hydraulics, and engineering. Understanding hydrostatic pressure is crucial for designing dams, underwater structures, and pressure vessels to ensure structural integrity and safety under varying fluid conditions.
Physical Origins: Vapour vs Hydrostatic Pressure
Vapour pressure originates from the evaporation of liquid molecules that escape into the gas phase, reflecting the equilibrium between liquid and vapor at a given temperature. Hydrostatic pressure results from the weight of a fluid column exerting force at a specific depth, governed by gravity and fluid density. Unlike hydrostatic pressure, which increases linearly with depth, vapour pressure depends solely on temperature and the inherent properties of the liquid.
Mathematical Expressions for Both Pressures
Vapour pressure (Pv) is mathematically expressed as Pv = P0 * exp(-DHvap / (R * T)), where P0 is a reference pressure, DHvap is the enthalpy of vaporization, R is the gas constant, and T is temperature in Kelvin. Hydrostatic pressure (Ph) is given by Ph = r * g * h, where r represents fluid density, g is gravitational acceleration, and h is the height of the fluid column. These equations quantify vapour pressure as a function of temperature-dependent phase change, while hydrostatic pressure depends on fluid depth and density.
Factors Influencing Vapour Pressure
Vapour pressure is influenced primarily by temperature, as increasing temperature raises the average kinetic energy of molecules, allowing more to escape from liquid to vapour phase. The chemical nature of the liquid, including intermolecular forces such as hydrogen bonding, significantly affects vapour pressure levels. Unlike hydrostatic pressure, which depends on fluid density and gravitational force, vapour pressure is independent of external pressure and solely determined by molecular interactions and temperature.
Determinants of Hydrostatic Pressure
Hydrostatic pressure depends primarily on fluid density, gravitational acceleration, and the height of the fluid column, following the equation P = rgh. This pressure increases linearly with depth in a fluid at rest and is influenced by the specific weight of the fluid and local gravity variations. Vapour pressure, in contrast, is determined by temperature and fluid properties, representing the equilibrium pressure exerted by a vapor above its liquid phase without reliance on fluid depth.
Practical Applications: Vapour Pressure in Industry
Vapour pressure is crucial in industries such as chemical manufacturing and food processing, where controlling evaporation rates and preventing equipment corrosion are essential for safety and product quality. It determines the boiling point of liquids under various conditions, influencing processes like distillation and solvent recovery. Hydrostatic pressure is less relevant in these applications, as it primarily affects the pressure exerted by fluid columns in engineering and fluid mechanics contexts.
Real-life Uses of Hydrostatic Pressure
Hydrostatic pressure, generated by the weight of a fluid column, is crucial in applications like water supply systems, dam engineering, and hydraulic lifts, where pressure at various depths ensures steady fluid flow and structural integrity. Unlike vapour pressure, which relates to phase changes and evaporation, hydrostatic pressure directly influences the design and safety of submerged structures and fluid containment. This pressure enables accurate measurement of liquid levels in tanks and reservoirs, ensuring effective fluid management in industrial and environmental settings.
Vapour Pressure vs Hydrostatic Pressure: Key Differences and Comparison
Vapour pressure represents the pressure exerted by a vapor in thermodynamic equilibrium with its liquid or solid phase at a given temperature, playing a crucial role in phase change and boiling phenomena. Hydrostatic pressure, on the other hand, is the pressure exerted by a fluid at rest due to the force of gravity, calculated as the product of fluid density, gravitational acceleration, and depth. Key differences highlight that vapour pressure depends primarily on temperature and the substance's properties, while hydrostatic pressure depends on fluid density and depth, affecting applications from weather prediction to underwater engineering.
Vapour pressure Infographic
 libterm.com
libterm.com