An isochoric process occurs when a gas is heated or cooled at constant volume, meaning no work is done since the container doesn't expand or contract. This process is characterized by changes in pressure and temperature while the volume remains fixed, making it essential for understanding thermodynamic cycles and heat transfer. Explore the rest of the article to deepen your understanding of how isochoric processes impact various engineering applications and everyday phenomena.
Table of Comparison
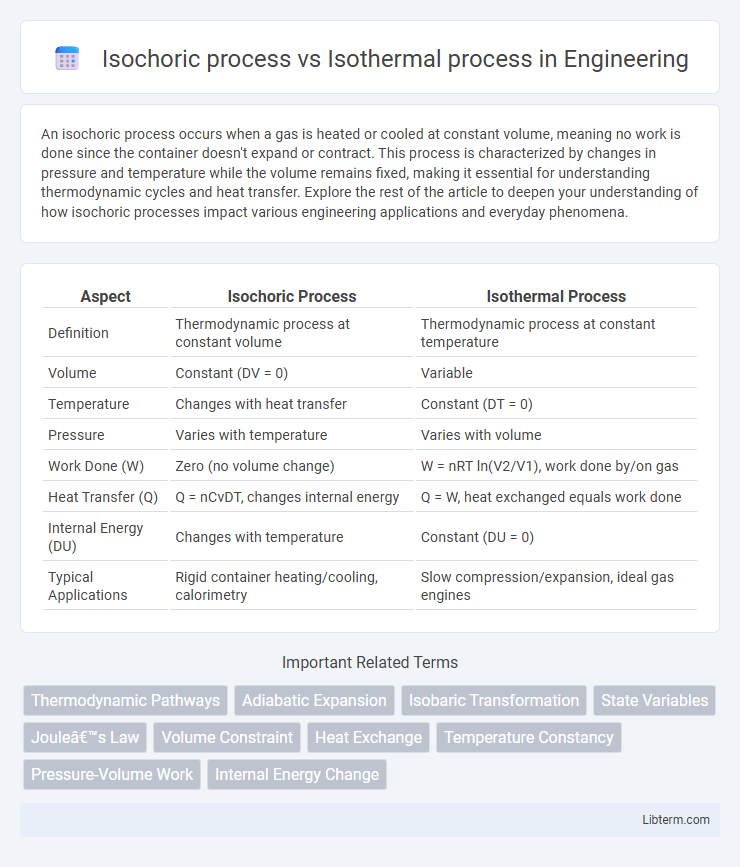
| Aspect | Isochoric Process | Isothermal Process |
|---|---|---|
| Definition | Thermodynamic process at constant volume | Thermodynamic process at constant temperature |
| Volume | Constant (DV = 0) | Variable |
| Temperature | Changes with heat transfer | Constant (DT = 0) |
| Pressure | Varies with temperature | Varies with volume |
| Work Done (W) | Zero (no volume change) | W = nRT ln(V2/V1), work done by/on gas |
| Heat Transfer (Q) | Q = nCvDT, changes internal energy | Q = W, heat exchanged equals work done |
| Internal Energy (DU) | Changes with temperature | Constant (DU = 0) |
| Typical Applications | Rigid container heating/cooling, calorimetry | Slow compression/expansion, ideal gas engines |
Introduction to Thermodynamic Processes
Isochoric process involves a constant volume, where no work is done and pressure changes with temperature, following the relation \( P \propto T \). Isothermal process maintains a constant temperature, resulting in variable volume and pressure changes that satisfy Boyle's law \( PV = \text{constant} \). Both processes are fundamental in thermodynamics for analyzing energy transfer and system behavior under specific constraints.
Defining Isochoric Process
An isochoric process occurs at constant volume, meaning the system's volume does not change while pressure and temperature may vary. In contrast, an isothermal process maintains a constant temperature, allowing volume and pressure to fluctuate. Understanding isochoric processes is essential in thermodynamics for analyzing systems like rigid containers where no work is done due to volume change.
Exploring Isothermal Process
The isothermal process maintains constant temperature throughout, allowing the gas to expand or compress while heat exchange balances work done by or on the system. Unlike the isochoric process, which occurs at constant volume and results in pressure changes due to temperature variations, the isothermal process follows Boyle's Law where pressure and volume have an inverse relationship. This characteristic makes the isothermal process crucial in thermodynamic cycles like the Carnot engine, maximizing efficiency by leveraging heat transfer under steady temperature conditions.
Key Differences Between Isochoric and Isothermal Processes
The isochoric process occurs at constant volume, meaning no work is done since the container's volume does not change, while the isothermal process occurs at constant temperature, allowing the gas to expand or compress with work done but internal energy remaining constant. In an isochoric process, pressure changes proportionally to temperature changes according to Gay-Lussac's law, whereas in an isothermal process, pressure inversely varies with volume following Boyle's law. Energy transfer in isochoric processes is solely as heat affecting internal energy, whereas isothermal processes involve heat exchange that balances work done to maintain constant temperature.
Pressure-Volume Relationship in Both Processes
In an isochoric process, the volume remains constant while pressure varies directly with temperature according to Gay-Lussac's law, resulting in no pressure-volume work done since volume change is zero. Conversely, an isothermal process maintains constant temperature, so pressure inversely varies with volume following Boyle's law, expressed mathematically as \(PV = \text{constant}\). This inverse relationship in isothermal processes means that any decrease in volume causes a proportional increase in pressure, allowing continuous work output or input during compression or expansion.
Energy Transformation and Heat Exchange
An isochoric process occurs at constant volume, where no work is done as the gas cannot expand or compress, causing all heat added or removed to directly change the system's internal energy. In contrast, an isothermal process maintains constant temperature, meaning the internal energy remains unchanged while the heat exchanged is entirely converted to or from work done by the system. Energy transformation in an isochoric process involves heat altering internal energy exclusively, whereas in an isothermal process, heat exchange balances precisely with work, keeping internal energy steady.
Real-Life Examples of Isochoric and Isothermal Processes
In an isochoric process, the volume remains constant while the pressure and temperature change, exemplified by heating a sealed rigid container or the combustion in a diesel engine cylinder before the piston moves. The isothermal process maintains constant temperature as volume changes, such as the slow compression or expansion of gases in a piston cylinder during ideal gas law experiments or the functioning of a Stirling engine. Real-life applications highlight the significance of these thermodynamic processes in internal combustion engines, refrigeration cycles, and industrial gas storage systems.
Mathematical Representation and Graphical Analysis
The isochoric process maintains constant volume, described mathematically by \( V = \text{constant} \) and characterized by the equation \( P/T = \text{constant} \) based on Gay-Lussac's law, resulting in vertical lines on a PV diagram. The isothermal process occurs at constant temperature, represented by the equation \( PV = nRT \) or \( P = \frac{nRT}{V} \), producing hyperbolic curves in the PV graph indicating inverse pressure-volume relationship. Graphically, isochoric lines appear as vertical segments showing pressure changes, whereas isothermal curves slope downward, reflecting simultaneous changes in pressure and volume at steady temperature.
Practical Applications in Engineering and Science
Isochoric process is commonly applied in pressure cookers and sealed gas containers where volume remains constant while pressure varies, enabling controlled reactions and energy transfer. Isothermal process is essential in refrigeration cycles and heat exchanger design, maintaining constant temperature to optimize thermal efficiency and minimize energy loss. Both processes are integral to thermodynamic systems analysis in engines and chemical reactors, influencing performance and safety parameters.
Conclusion: Choosing the Right Process
Selecting the appropriate thermodynamic process depends on the specific application requirements, where an isochoric process maintains constant volume resulting in pressure changes, while an isothermal process keeps temperature constant allowing heat exchange to balance work done. Isochoric processes are ideal in scenarios demanding volume stability, such as in rigid containers or certain engine cycles. Isothermal processes suit applications prioritizing temperature control, like slow compression in heat engines or refrigeration cycles, optimizing efficiency through heat transfer.
Isochoric process Infographic
 libterm.com
libterm.com