Solute refers to the substance dissolved in a solvent to form a solution, playing a crucial role in chemical mixtures and biological processes. Its concentration affects solution properties such as boiling point, freezing point, and osmotic pressure, essential for understanding reactions and formulations. Discover more about solute types, behaviors, and applications in the rest of this article to enhance your knowledge.
Table of Comparison
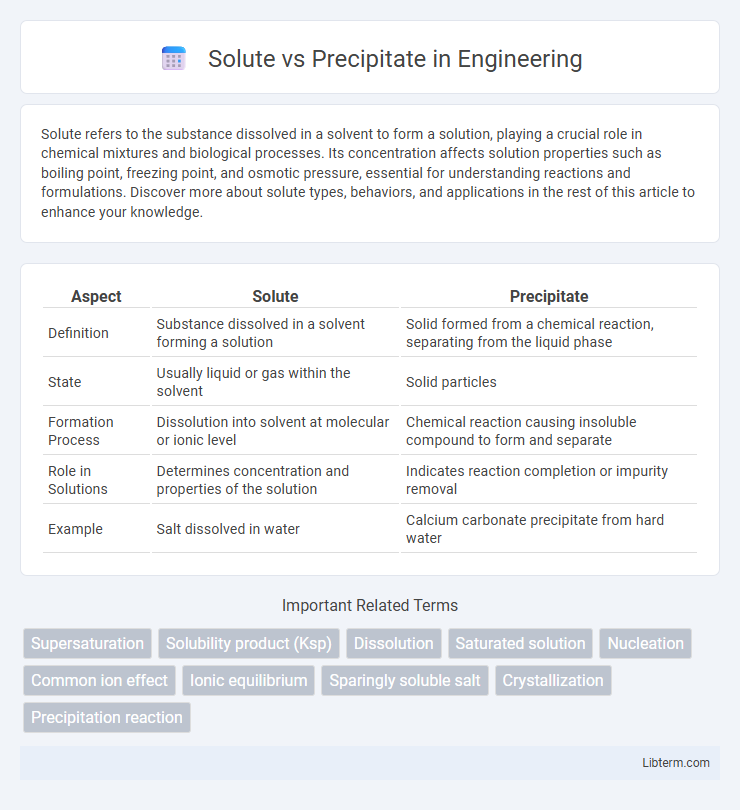
| Aspect | Solute | Precipitate |
|---|---|---|
| Definition | Substance dissolved in a solvent forming a solution | Solid formed from a chemical reaction, separating from the liquid phase |
| State | Usually liquid or gas within the solvent | Solid particles |
| Formation Process | Dissolution into solvent at molecular or ionic level | Chemical reaction causing insoluble compound to form and separate |
| Role in Solutions | Determines concentration and properties of the solution | Indicates reaction completion or impurity removal |
| Example | Salt dissolved in water | Calcium carbonate precipitate from hard water |
Understanding Solute and Precipitate: Key Differences
A solute is a substance dissolved in a solvent to form a homogeneous solution, while a precipitate is a solid that forms and separates from a solution during a chemical reaction. The solute exists in a dissolved state at the molecular or ionic level, whereas a precipitate appears as an insoluble solid resulting from the reaction of solutes. Understanding these key differences is crucial in fields like chemistry and environmental science, where solubility and precipitation impact reaction outcomes and material properties.
Defining Solutes: Meaning and Examples
Solutes are substances dissolved in a solvent to form a homogeneous mixture known as a solution, playing a crucial role in chemical processes. Common examples include salt dissolved in water and sugar mixed into tea, where the solute particles disperse uniformly throughout the solvent. Understanding solutes is essential for applications in chemistry, biology, and environmental science, as they influence concentration, reaction rates, and solution properties.
What is a Precipitate? Definition and Occurrence
A precipitate is a solid substance that forms and separates from a liquid solution during a chemical reaction. It occurs when the concentration of certain ions exceeds their solubility limit, causing insoluble compounds to crystallize and settle out of the solution. Precipitates are commonly observed in double displacement reactions, where the insoluble product appears as a cloudy or solid residue.
Role of Solutes in Chemical Solutions
Solutes play a crucial role in chemical solutions by dissolving in solvents to form homogeneous mixtures, influencing properties such as boiling point, freezing point, and electrical conductivity. They affect the solution's concentration, which determines the rate of chemical reactions and equilibrium states. Unlike precipitates, which are insoluble solids that separate out, solutes remain dissolved, enabling various chemical processes and applications including reactions, extractions, and industrial formulations.
How Precipitates Form: Precipitation Reactions Explained
Precipitates form during precipitation reactions when two soluble ionic compounds in aqueous solutions react to produce an insoluble solid, called the precipitate. This process occurs because the product ions combine to create a compound with low solubility, causing it to separate from the solution as a solid. Factors affecting precipitate formation include ion concentration, temperature, and the solubility product constant (Ksp) of the potential precipitate.
Factors Affecting Solubility and Precipitation
Solubility and precipitation depend heavily on factors such as temperature, pressure, and the nature of the solvent and solute. Increased temperature often enhances solubility of solids in liquids but decreases gas solubility, while higher pressure generally increases gas solubility in liquids. The ionic strength of the solution and the presence of common ions also significantly influence precipitation by shifting equilibrium positions according to the common ion effect.
Visual and Experimental Identification of Solute vs Precipitate
Solutes dissolve uniformly in solvents, producing a clear, homogeneous solution without visible particles, while precipitates form solid particles that appear as cloudy, suspended flakes or sediment during chemical reactions. Visual identification relies on observing solution clarity versus turbidity or solid formation, whereas experimental methods include filtration, centrifugation, or qualitative analysis tests to isolate and confirm the presence of precipitates. Spectroscopic and microscopic techniques further distinguish dissolved solutes from insoluble precipitates by analyzing particle size, concentration, and phase state.
Real-World Applications of Solutes and Precipitates
Solutes are crucial in pharmaceutical formulations where precise solubility ensures effective drug delivery and bioavailability, while precipitates play a key role in water treatment processes by removing harmful contaminants through chemical precipitation. In agriculture, solute nutrients dissolved in irrigation water enhance plant growth, whereas precipitate formation helps remediate soil by immobilizing toxic metals. Industrial metallurgy relies on controlling solute concentrations in alloys for desired material properties, and precipitate hardening strengthens metals by forming fine precipitates within the metal matrix.
Common Misconceptions: Solute vs Precipitate
A common misconception is that a solute and a precipitate are interchangeable terms, but a solute is a substance dissolved in a solvent forming a solution, while a precipitate is a solid that forms and separates from a solution during a chemical reaction. In solutions, solutes are present at the molecular or ionic level, whereas precipitates are visible solid particles that result from supersaturation or chemical changes. Understanding the distinct roles of solutes and precipitates is essential in fields like chemistry and environmental science where solution dynamics influence reaction outcomes and material properties.
Summary Table: Comparing Solute and Precipitate
A solute is a substance dissolved in a solvent to form a homogeneous solution, while a precipitate is an insoluble solid formed during a chemical reaction. Solutes disperse at the molecular or ionic level, resulting in transparent solutions, whereas precipitates consist of particles that aggregate and separate from the solution. Key differences include solubility, phase state, and appearance, with solutes remaining dissolved and precipitates visible as solid deposits.
Solute Infographic
 libterm.com
libterm.com