Boiling is the process where a liquid reaches its boiling point and rapidly changes into vapor, essential for cooking and sterilization. It plays a vital role in heat transfer and helps eliminate harmful bacteria from food and water. Discover more about how boiling affects everyday cooking and safety in the rest of this article.
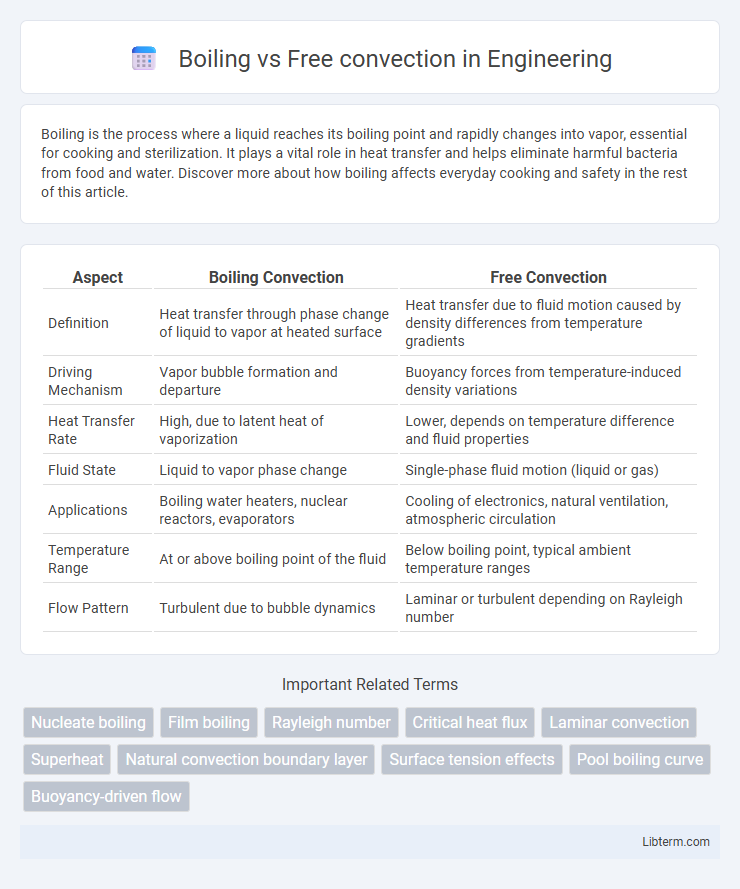
Table of Comparison
| Aspect | Boiling Convection | Free Convection |
|---|---|---|
| Definition | Heat transfer through phase change of liquid to vapor at heated surface | Heat transfer due to fluid motion caused by density differences from temperature gradients |
| Driving Mechanism | Vapor bubble formation and departure | Buoyancy forces from temperature-induced density variations |
| Heat Transfer Rate | High, due to latent heat of vaporization | Lower, depends on temperature difference and fluid properties |
| Fluid State | Liquid to vapor phase change | Single-phase fluid motion (liquid or gas) |
| Applications | Boiling water heaters, nuclear reactors, evaporators | Cooling of electronics, natural ventilation, atmospheric circulation |
| Temperature Range | At or above boiling point of the fluid | Below boiling point, typical ambient temperature ranges |
| Flow Pattern | Turbulent due to bubble dynamics | Laminar or turbulent depending on Rayleigh number |
Introduction to Boiling and Free Convection
Boiling is a phase-change heat transfer process where liquid rapidly transforms into vapor at a specific temperature, enhancing heat transfer through bubble formation and departure. Free convection occurs in fluids due to natural density differences caused by temperature gradients, driving fluid motion without external forces or pumps. Both boiling and free convection are critical mechanisms in thermal management systems, each optimizing heat dissipation based on fluid dynamics and temperature conditions.
Fundamental Principles of Heat Transfer
Boiling involves phase change heat transfer where liquid transforms into vapor at heated surfaces, significantly enhancing heat removal due to latent heat absorption. Free convection relies on buoyancy-driven fluid motion caused by temperature-induced density variations, facilitating heat transfer without external forces. The fundamental principle distinguishes boiling's combined conduction and latent heat mechanisms from free convection's reliance on natural fluid circulation for thermal energy transport.
Defining Boiling: Mechanisms and Types
Boiling is a heat transfer process involving phase change from liquid to vapor, characterized by the formation of vapor bubbles at nucleation sites on a heated surface. Types of boiling include nucleate boiling, where vapor bubbles form and detach vigorously, and film boiling, where a stable vapor layer insulates the surface, reducing heat transfer efficiency. Mechanisms driving boiling involve heat flux surpassing the liquid's saturation point, leading to rapid vaporization and enhanced convective heat transfer compared to free convection.
Understanding Free Convection: Natural Heat Movement
Free convection, also known as natural convection, occurs when fluid motion is driven solely by buoyancy forces resulting from density variations caused by temperature gradients. This process enables heat transfer without external mechanical forces, relying on the natural rise of warmer, less dense fluid and the descent of cooler, denser fluid. Understanding free convection is essential in engineering applications like HVAC system design, electronic cooling, and environmental heat transfer analysis.
Key Differences Between Boiling and Free Convection
Boiling involves phase change where a liquid transforms into vapor at its boiling point, creating bubbles, whereas free convection is the heat transfer through fluid motion caused by density differences without phase change. Boiling typically occurs at higher heat flux and involves latent heat transfer, while free convection relies on temperature gradients within the fluid to induce natural circulation. The heat transfer coefficient in boiling is significantly higher compared to free convection due to the vigorous mixing and phase change effects.
Factors Affecting Boiling and Free Convection Rates
Boiling rates are primarily influenced by surface temperature, fluid properties such as viscosity and thermal conductivity, and pressure, with nucleation site density playing a critical role in bubble formation. Free convection rates depend on fluid density differences caused by temperature gradients, fluid viscosity, and the geometry of the heated surface, which affect buoyancy-driven flow strength. Both processes are strongly affected by heat flux and boundary layer characteristics, determining the efficiency of heat transfer in thermal systems.
Practical Applications in Industry
Boiling is widely utilized in industrial heat exchangers and power plants for its high heat transfer rates during phase change processes, maximizing efficiency in steam generation and cooling systems. Free convection is critical in processes such as cooling electronic equipment, natural ventilation in buildings, and chemical reactors where fluid motion caused by temperature gradients facilitates heat dissipation without external pumps. Understanding the distinct mechanisms and heat transfer coefficients of boiling and free convection enables engineers to optimize thermal management systems, enhancing energy savings and operational reliability in various manufacturing sectors.
Advantages and Limitations of Each Method
Boiling offers rapid heat transfer with high heat flux, making it efficient for cooling high-power devices, but it requires precise control to avoid dry-out and burnout risks. Free convection provides passive cooling without mechanical parts, ensuring reliable operation with low energy consumption, though it delivers significantly lower heat transfer rates compared to boiling. Selecting between boiling and free convection depends on application-specific requirements, balancing heat transfer efficiency and system complexity.
Safety Considerations in Heat Transfer Processes
Boiling involves phase change heat transfer with rapid vapor generation, posing risks of surface burnout, coolant depletion, and pressure surges requiring robust system design and pressure relief mechanisms. Free convection relies on natural buoyancy-driven fluid motion without phase change, minimizing sudden thermal stresses but requiring careful temperature gradient control to prevent localized overheating. Safety in boiling systems demands precise monitoring of saturation temperature and pressure, while free convection systems emphasize stable temperature gradients and adequate fluid flow to avoid hot spots.
Conclusion: Choosing the Right Method for Heat Transfer
Boiling provides a highly efficient heat transfer method due to the latent heat of vaporization, making it ideal for applications requiring rapid heat removal. Free convection relies on natural fluid motion caused by temperature-induced density differences, offering a simpler but less intense heat transfer mechanism. Selecting between boiling and free convection depends on factors such as required heat flux, system complexity, and operating conditions, with boiling favored for high heat transfer rates and free convection suited for passive, low-maintenance designs.
Boiling Infographic
 libterm.com
libterm.com