Wetting describes the process by which a liquid spreads across or adheres to a solid surface, influenced by surface tension and contact angle. Understanding wetting properties is crucial in industries such as coatings, printing, and lubrication, where precise control over liquid behavior impacts product quality. Explore the rest of the article to learn how optimizing wetting can enhance your applications and processes.
Table of Comparison
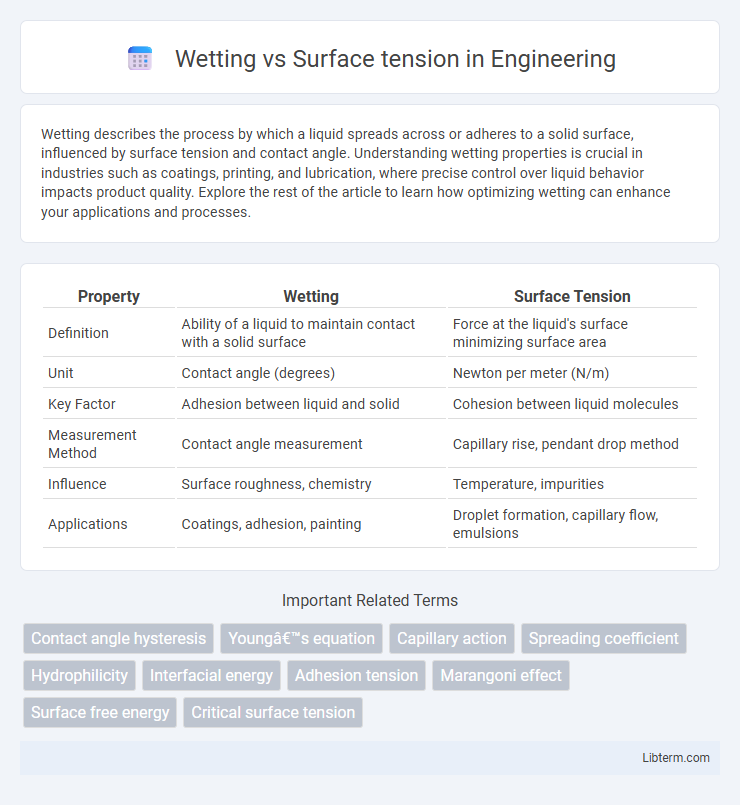
| Property | Wetting | Surface Tension |
|---|---|---|
| Definition | Ability of a liquid to maintain contact with a solid surface | Force at the liquid's surface minimizing surface area |
| Unit | Contact angle (degrees) | Newton per meter (N/m) |
| Key Factor | Adhesion between liquid and solid | Cohesion between liquid molecules |
| Measurement Method | Contact angle measurement | Capillary rise, pendant drop method |
| Influence | Surface roughness, chemistry | Temperature, impurities |
| Applications | Coatings, adhesion, painting | Droplet formation, capillary flow, emulsions |
Introduction to Wetting and Surface Tension
Wetting describes how a liquid interacts with and spreads over a solid surface, primarily determined by the balance of adhesive and cohesive forces. Surface tension arises from the cohesive forces between liquid molecules at the interface, causing the liquid surface to behave like a stretched elastic membrane. Understanding the relationship between wetting and surface tension is crucial for applications in coatings, printing, and material science.
Defining Wetting: Key Concepts
Wetting describes the ability of a liquid to maintain contact with a solid surface, influenced by intermolecular interactions at the interface. It is quantified by the contact angle, where lower angles indicate better wetting and higher angles display poor wetting or hydrophobicity. Surface tension plays a crucial role by resisting the deformation of the liquid interface, impacting how the liquid spreads or beads on the surface.
Understanding Surface Tension
Surface tension is the cohesive force at a liquid's surface caused by intermolecular attractions, creating a "skin" that resists external force. Understanding surface tension involves analyzing the balance between adhesive forces between the liquid and solid surfaces and cohesive forces within the liquid, which determines wetting behavior. Measuring surface tension using methods like the Wilhelmy plate or pendant drop technique provides quantitative insight critical for applications in coatings, detergents, and biomedical devices.
Molecular Interactions Behind Wetting
Wetting is governed by molecular interactions between a liquid and a solid surface, primarily involving adhesive forces that attract liquid molecules to the surface, overcoming cohesive forces within the liquid itself. The balance between these adhesive and cohesive interactions determines the contact angle, influencing how well a liquid spreads or beads on a surface. Surface tension arises from cohesive forces among liquid molecules at the interface, creating a minimized surface area, but wetting specifically describes how these molecules interact with and adhere to a solid substrate.
Factors Influencing Surface Tension
Surface tension is primarily influenced by temperature, with higher temperatures decreasing intermolecular forces and reducing surface tension. The presence of surfactants or impurities can significantly alter surface tension by disrupting cohesive forces at the liquid interface. Molecular structure and polarity also play key roles, as liquids with stronger hydrogen bonding typically exhibit higher surface tension.
Measurement Methods for Wetting and Surface Tension
Measurement methods for wetting commonly involve contact angle goniometry, where the angle formed between a liquid droplet and solid surface quantifies wettability. Surface tension is typically measured using techniques such as the Du Nouy ring method, Wilhelmy plate method, or pendant drop analysis, each providing precise values of liquid interface tension. Both methods rely on accurate imaging and force measurement tools to analyze liquid-solid or liquid-air interactions critical for material science and industrial applications.
Applications of Wetting in Industry
Wetting plays a critical role in industries such as coating, printing, and adhesives by determining how liquids spread on solid surfaces, directly impacting product quality and performance. Surface tension influences wetting behavior; lower surface tension enhances liquid spreading, improving processes like painting, lubrication, and cleaning. Optimizing wetting properties enables manufacturers to achieve uniform coatings, better adhesion, and efficient material interactions in applications ranging from electronics to pharmaceuticals.
Surface Tension in Everyday Life
Surface tension, a cohesive force at the liquid's surface, plays a critical role in everyday phenomena such as the formation of water droplets, the ability of insects like water striders to walk on water, and the capillary action that aids in plant water absorption. This surface property is essential for processes like soap foaming and the stability of bubbles, influencing cleaning efficiency and recreational activities. Understanding surface tension helps optimize industrial applications including inkjet printing, painting, and coating technologies.
Relationship Between Wetting and Surface Tension
Wetting is influenced by the surface tension of a liquid, which determines the liquid's ability to spread on or adhere to a solid surface. Lower surface tension typically enhances wetting by allowing the liquid to spread more easily, resulting in a smaller contact angle between the liquid and the solid. The balance between adhesive forces (liquid-solid interactions) and cohesive forces (liquid-liquid interactions) defines both the degree of wetting and the surface tension.
Future Trends and Research in Wetting and Surface Tension
Future trends in wetting and surface tension research emphasize the development of advanced surface coatings with tunable wettability for applications in energy harvesting, biomedical devices, and environmental remediation. Nanostructured materials and responsive polymers are being engineered to precisely control interfacial interactions, enabling breakthroughs in self-cleaning surfaces and droplet manipulation. Emerging computational models and real-time imaging techniques are accelerating the understanding of dynamic wetting phenomena at micro- and nanoscale levels.
Wetting Infographic
 libterm.com
libterm.com