Electroplating enhances the surface properties of metals by depositing a thin layer of material through electrochemical processes, improving corrosion resistance, wear protection, and aesthetic appeal. This technique finds widespread use in industries ranging from automotive to electronics, ensuring durability and functionality of components. Explore the detailed insights and applications of electroplating to understand how it can benefit your projects.
Table of Comparison
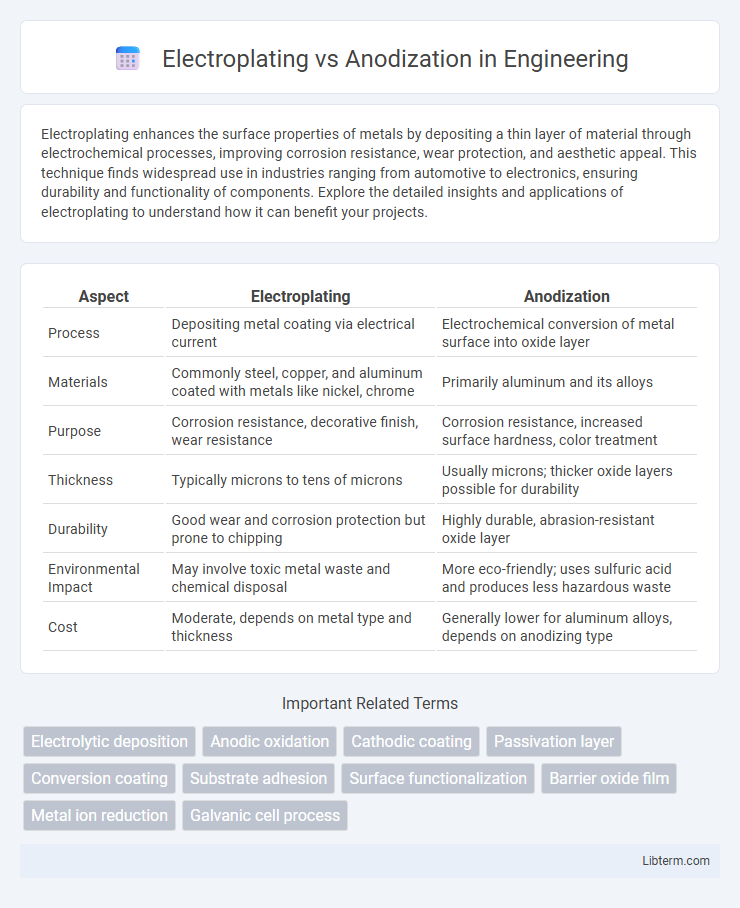
| Aspect | Electroplating | Anodization |
|---|---|---|
| Process | Depositing metal coating via electrical current | Electrochemical conversion of metal surface into oxide layer |
| Materials | Commonly steel, copper, and aluminum coated with metals like nickel, chrome | Primarily aluminum and its alloys |
| Purpose | Corrosion resistance, decorative finish, wear resistance | Corrosion resistance, increased surface hardness, color treatment |
| Thickness | Typically microns to tens of microns | Usually microns; thicker oxide layers possible for durability |
| Durability | Good wear and corrosion protection but prone to chipping | Highly durable, abrasion-resistant oxide layer |
| Environmental Impact | May involve toxic metal waste and chemical disposal | More eco-friendly; uses sulfuric acid and produces less hazardous waste |
| Cost | Moderate, depends on metal type and thickness | Generally lower for aluminum alloys, depends on anodizing type |
Introduction to Electroplating and Anodization
Electroplating involves depositing a thin metal layer onto a substrate through an electrochemical process, enhancing corrosion resistance and aesthetic appeal. Anodization, primarily used on aluminum, oxidizes the metal surface to create a durable, corrosion-resistant oxide layer that can be dyed in various colors. Both techniques improve surface properties but differ in their methods and types of protective layers formed.
Process Overview: Electroplating Explained
Electroplating involves depositing a thin layer of metal onto a substrate through an electrolytic cell, where the object serves as the cathode and metal ions from the anode solution are reduced and adhere to the surface. The process enhances corrosion resistance, improves appearance, and provides wear protection by creating a uniform metallic coating. Common electroplating metals include chromium, nickel, copper, and gold, which are selected based on the desired properties of the finished product.
Understanding the Anodization Process
Anodization is an electrochemical process that converts the metal surface into a durable, corrosion-resistant oxide layer, primarily applied to aluminum. This oxide layer enhances surface hardness and provides excellent protection against wear while allowing for vibrant color finishes through dye absorption. Compared to electroplating, which deposits a metal coating onto a substrate, anodization modifies the metal itself, resulting in a more integral and long-lasting protective barrier.
Key Differences Between Electroplating and Anodization
Electroplating involves depositing a metal layer onto a substrate through an electrochemical process to enhance corrosion resistance and aesthetic appeal, while anodization creates a thicker, protective oxide layer on metals like aluminum by controlled oxidation. Electroplating adds a foreign metal coating that can improve conductivity and wear resistance, whereas anodization alters the metal's surface itself, increasing hardness and providing a porous layer suitable for dyeing. The primary distinction lies in electroplating's application of an external metallic layer versus anodization's modification and thickening of the existing oxide layer for surface protection and durability.
Material Compatibility: Suitable Metals for Each Method
Electroplating is suitable for metals such as steel, copper, nickel, zinc, and brass, providing a protective or decorative coating by depositing a metal layer through an electric current. Anodization primarily applies to aluminum, titanium, and magnesium, enhancing corrosion resistance and surface hardness by forming a controlled oxide layer. Material compatibility significantly influences the choice of finishing method, with electroplating offering versatile substrate options while anodization is typically limited to valve metals.
Surface Finish and Appearance Comparison
Electroplating delivers a smooth, reflective surface finish by depositing a thin metal layer, often enhancing brightness and corrosion resistance with metals like chromium or nickel. Anodization creates a durable, matte or glossy oxide layer on aluminum surfaces, offering enhanced color options and excellent wear resistance without altering the base metal's texture significantly. While electroplating provides a metallic shine, anodization is favored for vibrant, long-lasting color and improved surface hardness in applications such as architectural panels and consumer electronics.
Corrosion Resistance: Electroplating vs Anodization
Electroplating enhances corrosion resistance by depositing a protective metal layer, such as nickel or chromium, that shields the base material from environmental exposure. Anodization improves corrosion resistance by creating a durable oxide layer on metals like aluminum, which acts as a barrier against moisture and chemical attack. While electroplating relies on the integrity of the metallic coating, anodization forms a chemically bonded surface layer that typically offers superior adhesion and long-term durability.
Typical Applications in Industry
Electroplating is widely used in the automotive and electronics industries for corrosion resistance, decorative finishes, and improved electrical conductivity on metal components. Anodization primarily serves the aerospace, architecture, and consumer electronics sectors by enhancing surface durability, wear resistance, and aesthetic appeal of aluminum parts. Both processes optimize material performance but cater to different industrial requirements based on substrate and desired surface properties.
Environmental Impact and Sustainability
Electroplating involves depositing a metal coating using hazardous chemicals and heavy metals, which can generate toxic wastewater and pose significant environmental risks if not properly managed. Anodization creates a durable oxide layer through an electrolytic process that uses non-toxic substances, resulting in less hazardous waste and improved corrosion resistance, enhancing sustainability. Opting for anodization over electroplating reduces environmental pollution and supports eco-friendly manufacturing practices in metal finishing industries.
Choosing the Right Method: Factors to Consider
Selecting between electroplating and anodization depends on the desired metal finish, corrosion resistance, and application environment. Electroplating provides a conductive, decorative coating ideal for enhancing appearance and wear resistance on metals like steel or copper. Anodization is best suited for aluminum substrates, offering increased oxide layer thickness that improves durability, corrosion protection, and paint adhesion without compromising electrical conductivity.
Electroplating Infographic
 libterm.com
libterm.com