Passivation enhances the corrosion resistance of metals by forming a protective oxide layer that prevents environmental damage. This chemical treatment is essential for prolonging the lifespan of stainless steel and other alloys used in critical applications. Discover how passivation can safeguard your materials and improve durability throughout this article.
Table of Comparison
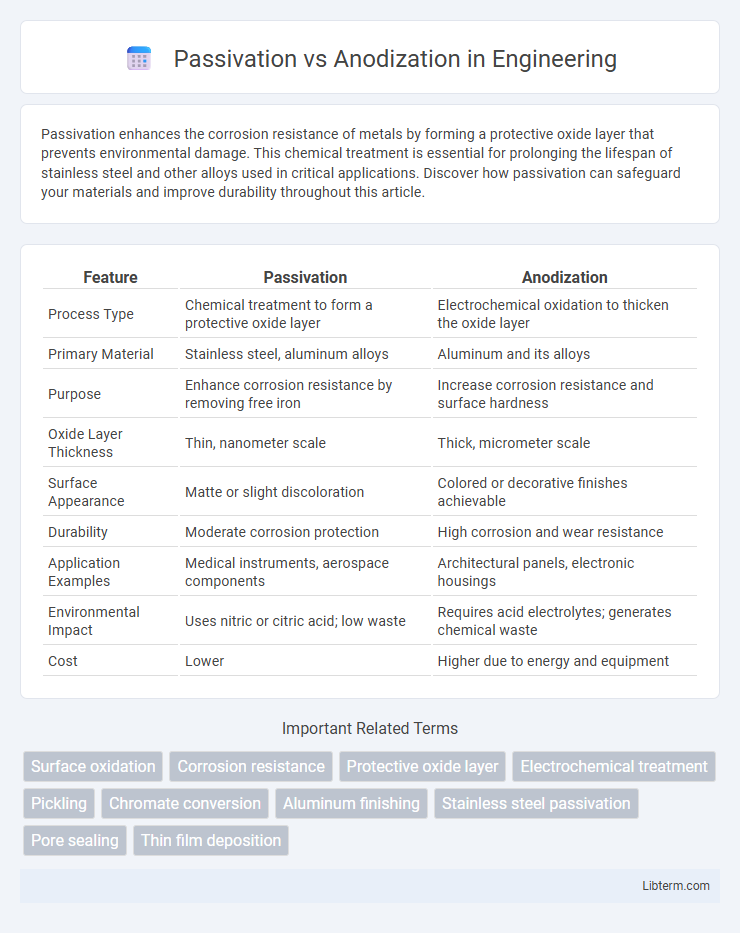
| Feature | Passivation | Anodization |
|---|---|---|
| Process Type | Chemical treatment to form a protective oxide layer | Electrochemical oxidation to thicken the oxide layer |
| Primary Material | Stainless steel, aluminum alloys | Aluminum and its alloys |
| Purpose | Enhance corrosion resistance by removing free iron | Increase corrosion resistance and surface hardness |
| Oxide Layer Thickness | Thin, nanometer scale | Thick, micrometer scale |
| Surface Appearance | Matte or slight discoloration | Colored or decorative finishes achievable |
| Durability | Moderate corrosion protection | High corrosion and wear resistance |
| Application Examples | Medical instruments, aerospace components | Architectural panels, electronic housings |
| Environmental Impact | Uses nitric or citric acid; low waste | Requires acid electrolytes; generates chemical waste |
| Cost | Lower | Higher due to energy and equipment |
Introduction to Passivation and Anodization
Passivation is a chemical treatment that enhances the corrosion resistance of metals, primarily stainless steel, by creating a thin, protective oxide layer on the surface. Anodization is an electrochemical process that thickens the natural oxide layer on metals such as aluminum, improving durability and aesthetic qualities. Both methods serve to protect metals but differ in application, thickness of the oxide layer, and the types of metals treated.
Understanding Passivation: Definition and Process
Passivation is a chemical treatment process that enhances the corrosion resistance of metals, particularly stainless steel, by forming a thin, protective oxide layer on the surface. This oxide layer prevents the metal from reacting with environmental elements, significantly reducing oxidation and rust formation. Unlike anodization, which involves an electrochemical process to thicken the oxide layer, passivation relies on chemical immersion in acidic solutions to remove free iron and impurities, thereby promoting the natural oxide layer's growth.
What is Anodization? Key Concepts Explained
Anodization is an electrochemical process that enhances the natural oxide layer on metal surfaces, primarily aluminum, by converting the metal surface into a durable, corrosion-resistant, and often decorative oxide coating. This controlled oxidation increases surface hardness, improves wear resistance, and allows for dye absorption, providing both functional and aesthetic benefits. Unlike passivation, which mainly removes contaminants to form a thin oxide layer, anodization actively thickens this layer to create a robust barrier against environmental damage.
Differences Between Passivation and Anodization
Passivation enhances corrosion resistance by forming a thin, inert oxide layer on the metal surface, primarily through chemical treatment, while anodization builds a thicker, porous oxide coating via electrochemical processes. Passivation is typically applied to stainless steel and other alloys to remove free iron and contaminants, whereas anodization is mainly used on aluminum and titanium to increase surface hardness and allow dye absorption. The key difference lies in the oxide layer's thickness and formation method, with anodization producing a more durable, functional surface compared to passivation's protective but thinner oxide film.
Applications of Passivation in Industry
Passivation is widely applied in the aerospace and electronics industries to enhance corrosion resistance by forming a thin oxide layer on stainless steel and other metals, protecting critical components from environmental degradation. It is essential in medical device manufacturing, where stainless steel implants and surgical tools require a biocompatible, corrosion-resistant surface to ensure patient safety and longevity. Industrial equipment in the chemical processing sector relies on passivation to prevent rust and corrosion, extending the lifespan of tanks, pipes, and reactors exposed to harsh chemicals.
Industrial Uses of Anodization
Anodization enhances corrosion resistance and surface hardness of metals, making it ideal for industrial applications in aerospace, automotive, and electronics industries. This electrochemical process creates a durable oxide layer on aluminum and other metals, improving wear resistance and enabling better adhesion for paints and adhesives. Industrial uses of anodization include protective coatings for aircraft components, automotive parts, electronic device housings, and architectural structures requiring long-lasting, aesthetically pleasing finishes.
Advantages and Limitations of Passivation
Passivation enhances corrosion resistance by forming a thin, protective oxide layer on stainless steel surfaces, reducing metal ion release and improving durability in aggressive environments. It offers cost-effective maintenance with minimal alteration to the metal's appearance but is limited by its reliance on inherently corrosion-resistant alloys and less effectiveness on damaged or contaminated surfaces. Passivation processes generally require controlled chemical treatment conditions, making them less versatile compared to anodization, which provides thicker oxide layers and enhanced wear resistance on aluminum and other non-ferrous metals.
Benefits and Drawbacks of Anodization
Anodization enhances corrosion resistance and surface hardness by creating a thick, durable oxide layer, ideal for aluminum alloys in aerospace and architectural applications. It improves aesthetic appeal through customizable color options and provides better adhesion for paints and adhesives but can increase surface brittleness and cause dimensional changes. The process requires careful control of parameters to avoid defects such as peeling or color inconsistencies, making it less suitable for complex geometries compared to passivation.
Choosing Between Passivation and Anodization
Choosing between passivation and anodization depends on the desired corrosion resistance and surface properties of the metal. Passivation enhances the natural oxide layer on stainless steel, improving its resistance to rust without altering the surface appearance. Anodization, primarily used for aluminum, creates a thicker, more durable oxide layer that provides both corrosion protection and aesthetic enhancement.
Future Trends in Surface Protection Methods
Future trends in surface protection methods emphasize advanced passivation techniques that enhance corrosion resistance by forming ultra-thin, self-healing oxide layers, while anodization evolves through the integration of nanostructured coatings to improve durability and aesthetic qualities. Emerging technologies incorporate environmentally friendly electrolytes and smart monitoring systems for real-time assessment of passivated and anodized surfaces, driving sustainable and efficient maintenance. The convergence of passivation and anodization processes with AI-driven predictive analytics is expected to revolutionize surface treatment applications in aerospace, automotive, and medical industries.
Passivation Infographic
 libterm.com
libterm.com