Chemical combustion is a rapid exothermic reaction between a fuel and an oxidant, typically oxygen, producing heat, light, and various reaction products such as carbon dioxide and water vapor. This process is fundamental in energy generation, powering engines, and industrial applications due to its efficiency and high energy output. Explore the rest of the article to understand the different types, mechanisms, and applications of chemical combustion in detail.
Table of Comparison
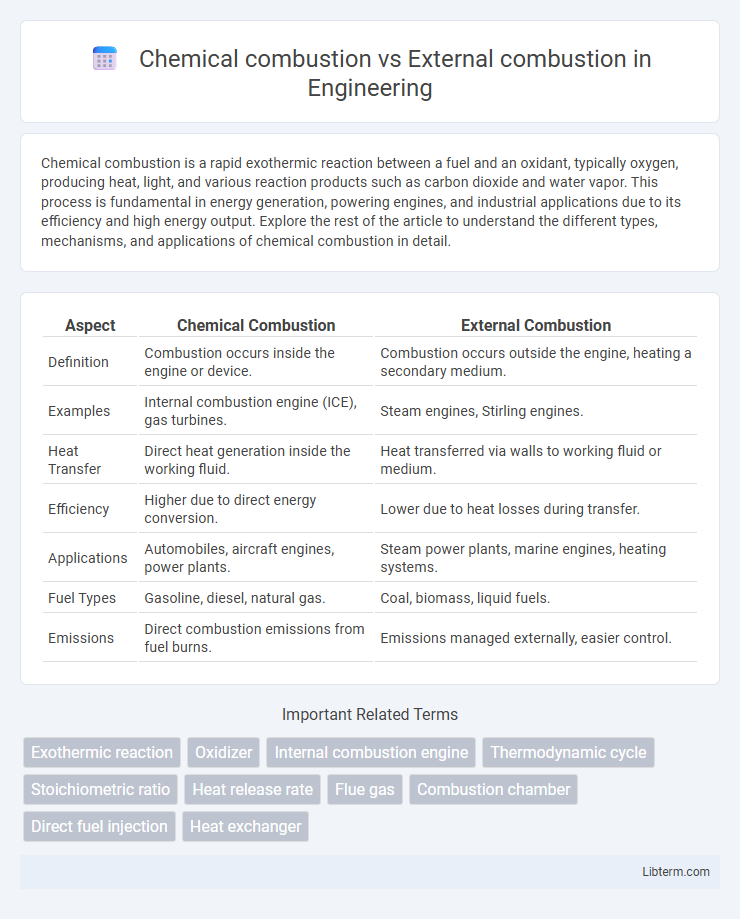
| Aspect | Chemical Combustion | External Combustion |
|---|---|---|
| Definition | Combustion occurs inside the engine or device. | Combustion occurs outside the engine, heating a secondary medium. |
| Examples | Internal combustion engine (ICE), gas turbines. | Steam engines, Stirling engines. |
| Heat Transfer | Direct heat generation inside the working fluid. | Heat transferred via walls to working fluid or medium. |
| Efficiency | Higher due to direct energy conversion. | Lower due to heat losses during transfer. |
| Applications | Automobiles, aircraft engines, power plants. | Steam power plants, marine engines, heating systems. |
| Fuel Types | Gasoline, diesel, natural gas. | Coal, biomass, liquid fuels. |
| Emissions | Direct combustion emissions from fuel burns. | Emissions managed externally, easier control. |
Introduction to Combustion Processes
Chemical combustion involves a rapid exothermic reaction between a fuel and an oxidizer, producing heat and light directly within the combustion chamber. External combustion occurs outside the system where the heat generated from burning fuel is transferred to a working fluid to perform mechanical work. Understanding these fundamental distinctions is crucial for optimizing energy conversion efficiency in engines and industrial processes.
Defining Chemical Combustion
Chemical combustion is a rapid exothermic reaction involving a fuel and an oxidant, typically oxygen, resulting in the release of heat and light. This process occurs within the fuel itself, generating energy through molecular oxidation. In contrast, external combustion involves burning fuel outside the engine or system, transferring heat to a working fluid for power generation.
Understanding External Combustion
External combustion involves the burning of fuel outside the engine, where heat generated transfers through a medium to produce mechanical energy, commonly seen in steam engines and power plants. This process allows for the use of various fuel types and promotes controlled combustion, reducing emissions compared to internal combustion engines. Understanding external combustion highlights its efficiency in heat management and its role in large-scale energy production.
Key Differences Between Chemical and External Combustion
Chemical combustion involves the direct oxidation of fuel within the combustion chamber, producing high-temperature gases that perform work, commonly found in internal combustion engines. External combustion occurs outside the working fluid system, where heat generated from burning fuel transfers through a surface or medium to produce steam or hot air for power, typical in steam engines. Key differences include the location of the combustion process, efficiency levels, and types of fuels used, with chemical combustion offering faster response and higher efficiency but external combustion providing cleaner emission control.
Chemical Reactions in Combustion
Chemical combustion involves exothermic reactions where fuel chemically combines with oxygen, producing heat, light, carbon dioxide, and water vapor, typically occurring within the combustion chamber of engines or burners. External combustion refers to processes where the combustion reaction occurs outside the working fluid, such as in steam engines or external furnaces, transferring heat through conduction or convection without direct mixing of fuel and working fluid. The chemical reactions in internal combustion are rapid and localized, while external combustion involves slower, controlled oxidation facilitating heat exchange to generate mechanical work or steam.
Examples of Chemical Combustion
Chemical combustion involves the rapid oxidation of fuels like gasoline, natural gas, and wood, producing heat and light in engines, stoves, and internal combustion vehicles. Examples include gasoline combusting in car engines to generate mechanical power and methane burning in residential heating systems. These processes release energy efficiently within enclosed chambers, contrasting with external combustion where heat is generated outside the working fluid.
Applications of External Combustion
External combustion engines are widely used in power plants where steam turbines convert heat from burning coal, oil, or biomass into electricity, optimizing large-scale energy production. These engines are also integral to marine propulsion in steamships, harnessing external heat sources for reliable long-distance travel. Industrial applications include combined heat and power (CHP) systems that enhance energy efficiency by utilizing waste heat for heating and mechanical work.
Efficiency and Energy Output Comparison
Chemical combustion engines, such as internal combustion engines, typically achieve higher thermal efficiency due to direct fuel burning inside the combustion chamber, resulting in more effective energy conversion and higher energy output per unit of fuel. External combustion engines, like steam engines, generally exhibit lower efficiency because heat transfer occurs indirectly through a heat exchanger, causing greater energy loss and reduced power generation. Advances in fuel technology and combustion chamber design continue to improve efficiency in chemical combustion systems, while external combustion methods remain limited by thermodynamic constraints and heat transfer inefficiencies.
Environmental Impact: Chemical vs External Combustion
Chemical combustion, typically occurring within engines or industrial processes, often produces higher levels of nitrogen oxides (NOx), carbon monoxide (CO), and particulate matter, contributing to significant air pollution and greenhouse gas emissions. External combustion, as seen in steam turbines powered by boilers, can utilize cleaner fuel sources such as biofuels or waste heat, reducing harmful emissions and improving overall environmental sustainability. The management of combustion byproducts and the choice of fuel greatly influence the ecological footprint of both chemical and external combustion systems.
Future Trends in Combustion Technologies
Emerging trends in chemical combustion focus on enhancing fuel efficiency and reducing emissions through advanced catalysts and alternative fuels like hydrogen and biofuels. External combustion technologies are evolving towards integration with renewable energy systems and carbon capture methods to improve sustainability in power generation. Innovations in materials and thermal management predict a shift to more resilient, low-emission combustion systems tailored for industrial and transportation sectors.
Chemical combustion Infographic
 libterm.com
libterm.com