A Frenkel defect occurs when an atom or ion is displaced from its regular lattice site to an interstitial site, creating a vacancy at the original position and an interstitial defect. This type of point defect affects the physical properties of materials, such as electrical conductivity and diffusion rates, especially in ionic crystals like silver halides. Explore the rest of the article to understand how Frenkel defects influence material behavior and practical applications.
Table of Comparison
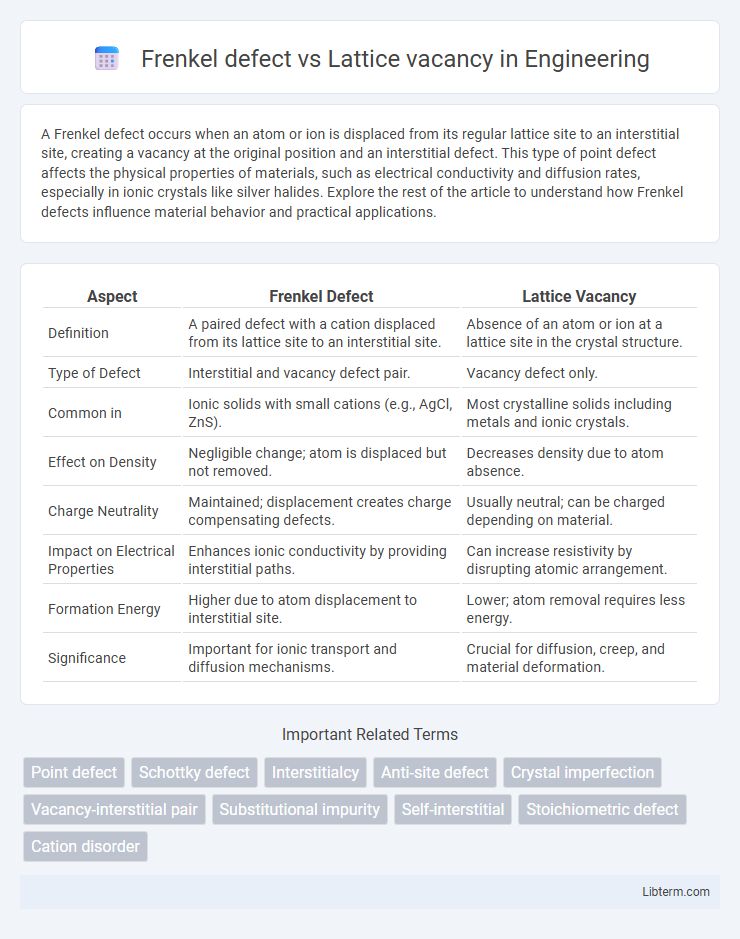
| Aspect | Frenkel Defect | Lattice Vacancy |
|---|---|---|
| Definition | A paired defect with a cation displaced from its lattice site to an interstitial site. | Absence of an atom or ion at a lattice site in the crystal structure. |
| Type of Defect | Interstitial and vacancy defect pair. | Vacancy defect only. |
| Common in | Ionic solids with small cations (e.g., AgCl, ZnS). | Most crystalline solids including metals and ionic crystals. |
| Effect on Density | Negligible change; atom is displaced but not removed. | Decreases density due to atom absence. |
| Charge Neutrality | Maintained; displacement creates charge compensating defects. | Usually neutral; can be charged depending on material. |
| Impact on Electrical Properties | Enhances ionic conductivity by providing interstitial paths. | Can increase resistivity by disrupting atomic arrangement. |
| Formation Energy | Higher due to atom displacement to interstitial site. | Lower; atom removal requires less energy. |
| Significance | Important for ionic transport and diffusion mechanisms. | Crucial for diffusion, creep, and material deformation. |
Introduction to Crystal Defects
Frenkel defect involves an atom displaced from its lattice site to an interstitial site, creating a vacancy and an interstitial defect pair within the crystal. Lattice vacancy refers to a missing atom or ion from its regular lattice position, resulting in an empty lattice site. Both Frenkel defects and lattice vacancies significantly influence material properties such as electrical conductivity, diffusion rates, and mechanical strength in crystalline solids.
Overview of Point Defects in Solids
Frenkel defects and lattice vacancies are fundamental types of point defects in crystalline solids that significantly influence material properties such as diffusion and electrical conductivity. A Frenkel defect occurs when an atom or ion is displaced from its lattice site to an interstitial position, creating a vacancy-interstitial pair, whereas a lattice vacancy consists solely of a missing atom or ion from the lattice site without an accompanying interstitial. Understanding these defects is crucial in tailoring the mechanical strength, ionic conductivity, and other physical properties of materials used in semiconductors, ceramics, and metals.
Defining the Frenkel Defect
A Frenkel defect occurs when an atom or ion is displaced from its original lattice site to an interstitial site, creating a vacancy-interstitial pair within the crystal structure. Unlike a lattice vacancy, which is simply a missing atom at a lattice site, the Frenkel defect involves both a vacant lattice position and an atom occupying a normally unoccupied interstitial position. This defect type is common in ionic crystals with significant size differences between ions, affecting properties such as ionic conductivity and diffusion.
Understanding Lattice Vacancy (Schottky Defect)
A lattice vacancy, known as a Schottky defect, occurs when an equal number of cations and anions are missing from their regular lattice sites, maintaining charge neutrality and density reduction in ionic crystals like NaCl. This defect type significantly influences the material's ionic conductivity, diffusion rates, and mechanical properties by creating empty lattice sites that facilitate ion migration. Unlike a Frenkel defect, where an ion is displaced to an interstitial site, Schottky defects involve the absence of ions, affecting the crystal's stoichiometry and often leading to decreased density and altered electrical characteristics.
Structural Differences: Frenkel vs. Lattice Vacancy
Frenkel defects involve an atom displaced from its original lattice site to an interstitial position, creating both a vacancy and an interstitial defect within the crystal structure. Lattice vacancies consist solely of missing atoms at specific lattice points without the presence of displaced interstitial atoms. The structural distinction lies in Frenkel defects causing a paired vacancy-interstitial configuration, while lattice vacancies represent isolated absence of atoms in the lattice framework.
Formation Mechanisms of Frenkel and Lattice Vacancies
Frenkel defects form when an atom or ion vacates its lattice site and occupies an interstitial position, creating a paired vacancy and interstitial defect without altering the overall stoichiometry. Lattice vacancies arise from thermal agitation or external influences, where atoms are simply missing from their regular lattice sites, disrupting the crystal structure. The energy required for Frenkel defect formation involves both the displacement to an interstitial site and vacancy creation, while lattice vacancy formation energy concerns only the removal of atoms from lattice positions.
Impact on Physical Properties of Materials
Frenkel defects create interstitial atoms and vacancies, leading to increased diffusion rates and altered electrical conductivity in ionic crystals. Lattice vacancies primarily cause shrinkage of the crystal lattice and can enhance ionic conductivity by providing pathways for ion migration. Both defects influence mechanical strength and diffusion but impact material properties differently due to the nature of atom displacement within the crystal structure.
Examples of Materials Exhibiting Each Defect
Frenkel defects commonly occur in ionic crystals such as silver halides (AgCl, AgBr) where cations are displaced from their lattice sites into interstitial positions, maintaining charge neutrality. Lattice vacancies are prevalent in metals like copper, aluminum, and gold, as well as ionic crystals such as sodium chloride (NaCl), where atoms are simply missing from their regular lattice sites. The presence of Frenkel defects in materials like ZnS and AgBr affects ionic conductivity significantly, while lattice vacancies in metals influence properties such as diffusion and mechanical strength.
Detection and Analysis Techniques
Frenkel defect detection often relies on techniques like positron annihilation spectroscopy and electron paramagnetic resonance to identify displaced ions within the crystal lattice. Lattice vacancies are commonly analyzed through X-ray diffraction and transmission electron microscopy, which reveal missing atoms and disruptions in lattice periodicity. Advanced atom probe tomography further enables 3D mapping of both Frenkel defects and vacancies at the atomic scale for precise characterization.
Comparative Summary: Frenkel Defect vs. Lattice Vacancy
Frenkel defects involve a cation displaced from its lattice site to an interstitial site, creating a vacancy-interstitial pair, whereas lattice vacancies refer to missing atoms at regular lattice positions without displacement. Frenkel defects typically occur in ionic crystals with small cations, enhancing ionic conductivity, while lattice vacancies are common in metals and affect mechanical properties like diffusion and creep. The formation energy for Frenkel defects is generally higher than that for lattice vacancies, influencing their respective concentrations and roles in material behavior.
Frenkel defect Infographic
 libterm.com
libterm.com